
Source: Pharmaceutical Proficient Bio
1、Preclinical research
1.Research and development (2-3 years)
Laboratory research to find new compounds with potential to treat specific diseases.
1)Drug target discovery and confirmation
This is the starting point for all work, and only when the target is determined, all subsequent work has a basis for development.
2)Screening and synthesis of compounds
According to the spatial structure of the target, a series of matching molecular structures are screened from the virtual compound library to synthesize these compounds, which are called lead compounds.
3)Validation and optimization of active compounds
Not all lead compounds can meet the requirements. At this stage, compounds with high activity and low toxicity need to be verified by in vitro cell tests, and their structures need to be optimized according to the structure-activity relationship. These compounds are called drug candidates. At the same time, there are also cases where A compound has no effect on the target A target, but may have very good activity on other B targets and C targets, which are not listed for the time being.
2、Preclinical trials (usually 2-4 years)
The purpose of this phase is to evaluate the pharmacological and toxicological effects of the drug, the absorption, distribution, metabolism and excretion of the drug (ADME). The second is the production process, quality control, stability and other research (CMC). The first part of the experiment needs to be carried out at the animal level, and the results of cell experiments and live animal experiments can sometimes be very different. The purpose of this step is to determine the effectiveness and safety of the drug.
The second part needs to be completed in a GMP compliant workshop.
1)Pharmacological study
Including: pharmacodynamics, pharmacokinetics
2)Toxicological study
Acute toxicity, chronic toxicity, reproductive toxicity, carcinogenesis, teratogenesis, mutagenicity
3)Formulation development
Formulation development is an important part of drug application. For example, some drugs are poorly absorbed orally and need to be developed as injections. Some drugs lose their activity in stomach acid and need to be developed into enteric preparations.
Some compounds do not dissolve well, which can also be partially solved by preparations. There is also the need for local administration, which needs to be developed into atomizing agents, poultices, etc.
2、临床试验审批 Investigational New Drug(IND)
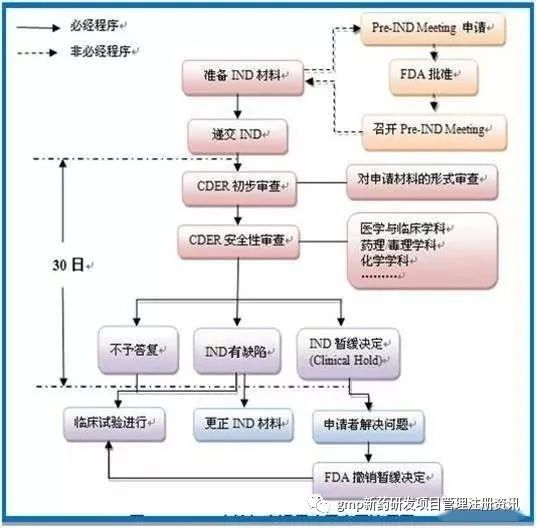
3、Clinical trials (typically 3-7 years)
The human trial was divided into three phases:
• Phase I clinical 20-100 cases, normal people, mainly to evaluate the safety.
• Phase II clinical 100-300 patients, mainly to evaluate the effectiveness.
• Phase III clinical 300-5000 patients, expand the sample size, further evaluation. Because phase I - III is very important in the overall drug development process, we will focus on this part. Traditionally, clinical studies of new drugs are divided into phase I/II/III, and later Phase II is divided into IIa and IIb (largely because of cancer drug research), followed by the concept of phase 0 studies (also largely because of cancer drug development).
Then it was proposed that phase 0/I was the early clinical study, IIa was the middle clinical study, and IIb and III were the late clinical study. This classification, in fact, I think is very important for us in clinical research design in two ways: 1. The stages are related to the purpose of the research, and different purposes can be divided into different periods/stages. 2. Clinical studies of different periods/stages often correspond to a certain research framework design (cross-study, parallel control, one-arm, endpoint selection, etc.). Therefore, the significance of understanding the different periods/stages of clinical research has gone beyond knowing that there is such a division itself. One end of the stage is the research purpose, and the other end is the corresponding design framework (of course, it is only a rough framework, and many problems should be determined according to diseases, drugs, etc.). Understanding the stage helps us to understand what design methods and ideas are in general for a research purpose, and how to deal with them in general. Why this should be done, and what are the advantages and disadvantages of various designs. So, let's first look at the purposes of the different stages of research.
Phase 1.0
Under the premise of meeting certain statistical requirements, with a limited sample size (usually no more than 20) and a limited time (the treatment period for each patient is usually no more than a few weeks), the purpose is to initially determine whether an investigational drug is effective, whether a certain dose is effective, and whether it should be continued to be developed, and to see whether some therapeutic evaluation methods are feasible. From the point of view of purpose, phase 0 study is also a preliminary judgment of drug effects, similar to phase II (especially IIa), so the design of phase 0 is similar to the one-arm study of IIa in some aspects, but due to the small sample size and to meet certain statistical requirements, it also has its own characteristics, which may be more complicated to design.
Phase 2.I
It is usually dose finding (dose-ranging), drug substitution (PK) and drug movement (PD), and the sample size is also small (generally no more than 20, or about 20). PD, in particular, usually uses cross-over studies because cross-over studies have their own characteristics and are suitable for such design purposes. In cross-over studies, each patient is his own control (in different stages, he plays the role of study group and control group respectively), and the variables of cross-over studies are generally smaller than those of parallel controls, so the efficiency of cross-over studies is much higher than that of parallel controls in terms of sample utilization. If 20 patients are included in cross-over studies, Parallel control of 50 cases may not be able to obtain similar results. However, cross-control also has many limitations. For example, the impact of shedding and loss of follow-up on cross-control studies is much greater than that of parallel control studies. The same person receives different treatments at different times, intermediate intervals for washout periods, resulting in aftereffects, and treatment-time interactions, etc. Therefore, in late-stage clinical research and development, useful cross-controls are rarely seen (there are, but far less than parallel controls).
Phase 3.II
Phase II is a preliminary evaluation of efficacy and safety (in fact, safety throughout development), so it is generally referred to as safety & activity studies (SA studies). There are various approaches. According to different clinical disease environments and previous data, single-arm, parallel control RCT can be used. The endpoint is generally surrogate, not clinical outcome. If IIa and IIb are divided, IIa is usually one-arm, and the sample size is generally not more than 100; IIb is often a parallel RCTS.
Phase 4.III
Phase III is a rigorous confirmatory clinical study that verifies the effect of a drug. Clinical studies are presented at many conferences, often using such studies as a model, and are presented under the framework of hypothesis testing.
4、New DrugApplication for new drug marketing approval
●NDA Application - CTD(Common Technical Document)
CTD is mainly composed of five modules:
①Administrative and regulatory information
②Overview: A high level of overview of drug quality, non-clinical, and clinical trials
③Drug quality specification
④Non-clinical study report
⑤Clinical study report process:
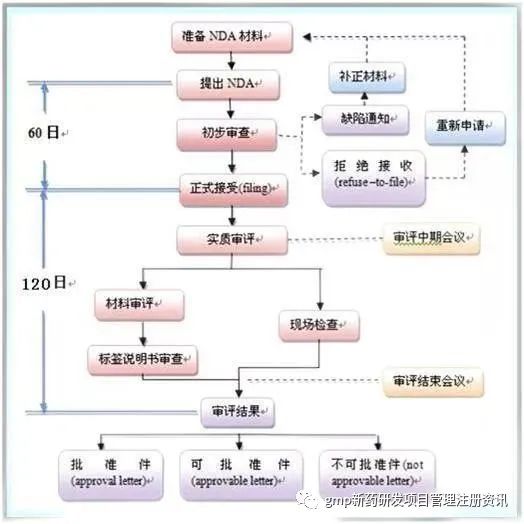
①Approval letter
Meet the requirements, can be listed
②Approvability letter
Basically meet the requirements, a few deficiencies can be modified.
The applicant shall respond to the amendment within 10 days of receipt, otherwise it will be deemed to be withdrawn automatically.
③Refusal letter
There are serious problems or a lot of additional information is needed.
The applicant may file an amendment within 10 days or request a hearing within 30 days.
●NDA special review procedure
①Priority Reviews
NDA reviews are prioritized for drugs that offer significant improvements in the treatment, diagnosis, or prevention of disease over drugs already on the market.
②Accelerated Approval
A drug product intended to treat a serious or life-threatening disease that has a reasonable and measurable "Surrogate endpoint", that is, an indicator of the drug's expected therapeutic effect, that ADAPTS the review criteria and uses the "surrogate endpoint" review.
③Fast-track
Drugs used to treat serious or life-threatening diseases that have the potential to meet clinical unmet medical needs, early intervention, close communication, and phased submission of declarations.
In the case of Vioxx, on November 23, 1998, Vioxx submitted an NDA application, number 21-042, "which was approved by the FDA on May 20, 1999, and took 178 days."
In addition, the full English version of ANDA is Abbreviated New Drug Application, and the Chinese version is called generic drug registration application. Generic drugs need to be identical to the marketed drug in terms of the substance, dosage form, specification, route of administration, and conditions of use (unless the same conditions of use cannot be achieved due to patent issues). Not only that, generic drugs also need to be consistent with the original drug in quality, effect and indication, so they are also called generic drugs or generic drugs. Because the manufacturer of the original drug has done relevant animal and human studies and demonstrated the efficacy and safety of the drug, the generic drug application does not need to repeat these experiments, only need to scientifically prove that the generic drug is bioequivalent to the original drug.
5、Post-marketing study
Clinical monitoring phase: Phase IV clinical
The subjects should be more than 2000, and the sociality should be examined.
Take Vioxx again: A gastrointestinal trial of VIGOR in 2000 showed fewer gastrointestinal side effects, but a doubling of the risk of heart attack/stroke after 18 months of use.
In 2001, the "APPROVe" adenomatous polyp prevention trial - a higher risk of cardiovascular disease after taking the drug for more than 18 months.
6、Approval after listing (generally 4-10 years after listing)
Objective: To review the efficacy and safety of NDAs.