
Source: Bairong Capital
1. Compared with traditional animal models, organoids have higher clinical relevance, and have great development potential in the field of drug development and tumor precision therapy.
2. In the field of drug research and development, the cost of organoids is similar to that of cell lines, but because of the advantages of high clinical relevance, the rounds of drug screening can be reduced, thus significantly reducing the capital cost and time cost of drug research and development, which is recognized by first-line drug developers and enterprises, and major pharmaceutical companies have actively laid out.
3. In the field of tumor precision diagnosis and treatment, industry-related regulations and guidelines are still in the process of formulation, and with the major scientific research institutions and enterprises to obtain more clinical trial data, the C-end scene as the largest part of the application of organoids will grow rapidly.
4.With reference to the development of the NGS market, manufacturers with only organoid culture service capabilities may fall into a situation of serious service homogenization and fierce competition in the future; Therefore, companies with a composite team background and the ability to develop and manufacture organoid devices, consumables and chips have more development potential.
01 Organoids definition and characteristics
Organoids are 3D cultured micro-organs with 3D structures that can mimic in vitro normal (or diseased) internal organs (or tissues). Organoids are not human organs in the real sense, but they can simulate real organs in structure and function, and can be long-term stable culture.
The source of organoids is usually human stem cells or tissue cells, which has a high degree of clinical relevance (that is, the response of organoids to drugs is more consistent with the response of humans to drugs than other models, such as mice), and the culture cost is low, so it has a huge application prospect in the field of scientific research, drug development and clinical precision medicine.
02 Organoid application and market
2.1 Organoids are used in drug development
In the process of drug development, organoids are mainly used for disease modeling, lead compound screening and efficacy detection in preclinical studies. Their advantages are high clinical relevance, relatively low cost, and efficient cultivation and subsequent analysis on a large scale. Under the background of high expenditure on new drug research and development and huge burden on new drug enterprises, organoids are expected to become an emerging solution to reduce cost and increase efficiency in the process of drug research and development.
2.1.1 The cost of drug research and development continues to rise, and the success rate of research and development continues to decline, and it is urgent to reduce cost and increase efficiency
The cost of capital and time for drug development continues to rise. The Tufts Centre analysed spending on 106 new drugs from 10 companies between 1995 and 2007 and found that the cost of developing new drugs was $1.39 billion, with capitalised spending exceeding $2.5 billion.
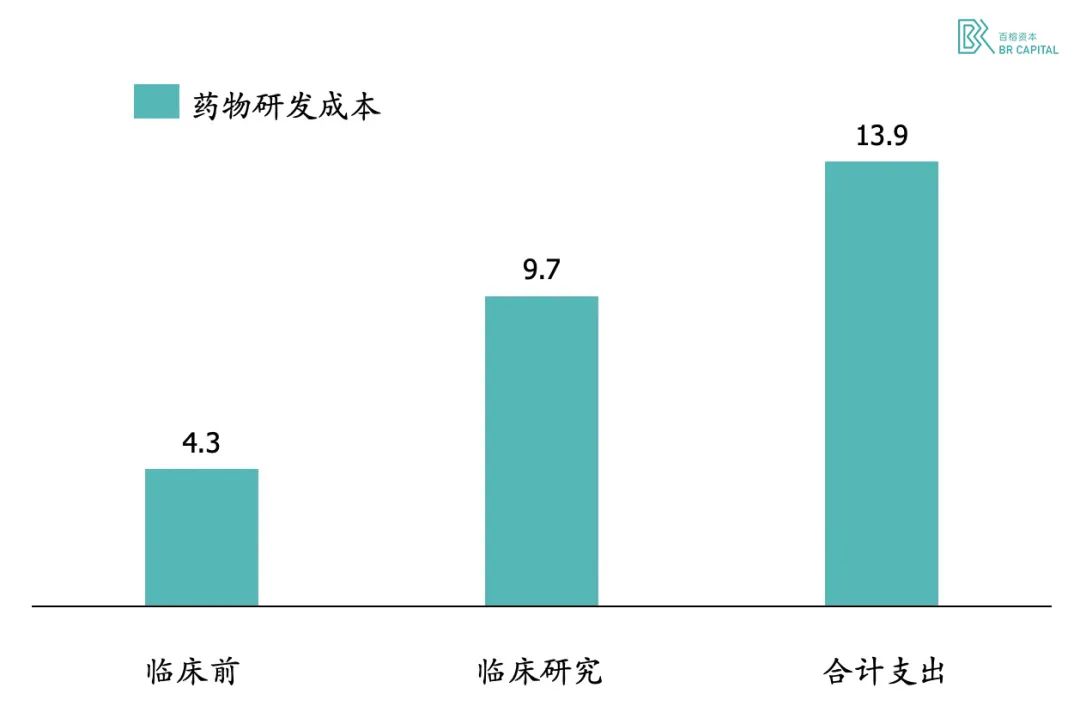
Exhibit 1. New drug research and development expenditure statistics (unit: USD 100 million) * Source: Tufts Centre
In terms of capitalized R&D costs, the preclinical structural optimization phase cost more than the Phase III clinical trial, which is probably far more than most people think.
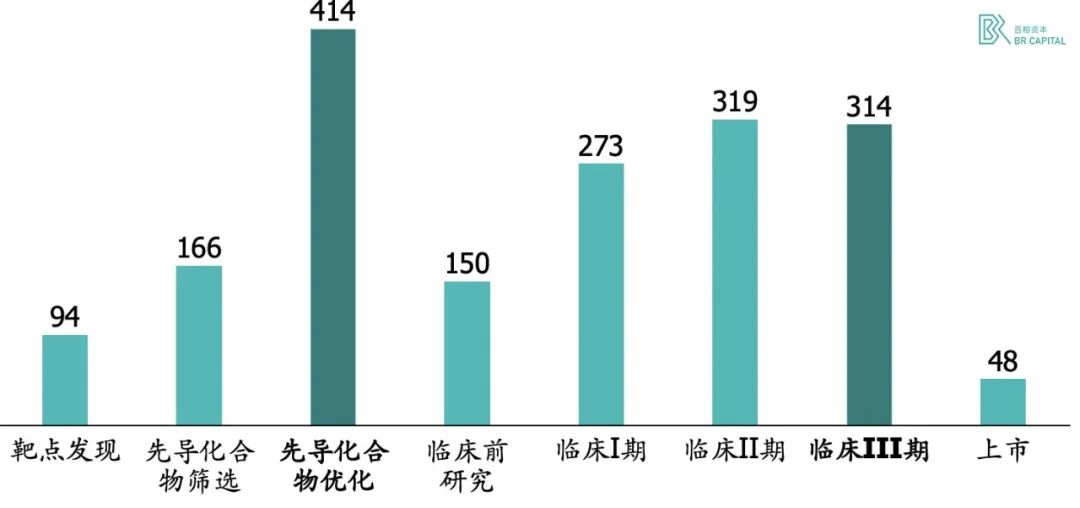
Exhibit 2. New drug research and development expenditure statistics (in millions of US dollars) * Source: 《How to improve R&D productivity: the pharmaceutical industry’s grand challenge》
In the field of cancer drug research, Vinay Prasad, a cancer doctor and researcher at the University of California, San Francisco, and others calculated the cost of developing cancer drugs. Between January 1, 2006 and December 31, 2015, the average research and development cost of the 10 FDA-approved anti-tumor drugs was $720 million, so there is a huge funding threshold in the field of new drug development.* Source: 《Research and Development Spending to Bring a Single Cancer Drug to Market and Revenues After Approval》
The time taken to develop new drugs has also raised eyebrows. Preclinical drug development takes an average of 4.5 years; The average drug development cycle from preclinical target screening to final marketing is at least 13.5 years.
The efficiency of clinical drug development is generally low
BioMedTracker analyzed 7,455 drug discovery projects that passed through the clinic between 2006 and 2015. The success probability of Phase I trials was 63%, Phase II trials 31%, and Phase III trials 58%, with an overall success rate of 9.6%. When analyzed by treatment area, the overall success rate ranged from 26% in the blood drug-related pipeline to 5% in the oncology pipeline.
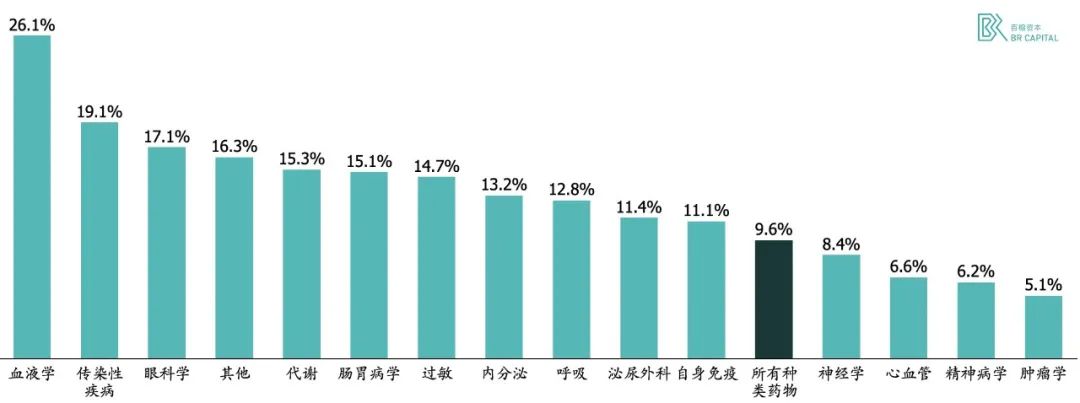
* Exhibit3. New drug research and development success rate statistics * Source: 《Clinical Development Success Rates 2006-2015》
L, head of research and development of the organoid department of internationally renowned pharmaceutical companies: We now have a lot of drug failures, sometimes it may fail in vitro tests, or it may be after in vitro tests, and then fail in animal experiments, and the overall success rate is not more than 10%.
2.1.2 Organoids can be used in drug development for disease modeling, drug screening and efficacy testing
1. The use of organoids in drug development
At present, the two main applications of organoids in drug research and development are drug screening and efficacy testing. Organoids can be differentiated into mini tissues of various parts of the human body through stem cells, and drug suppliers conduct drug research and development; It can also rely on the high clinical relevance of organoids for high-throughput screening of millions of drugs; Compounds and cell therapies can be tested for efficacy. At present, the main research direction of major pharmaceutical companies is to use organoids for drug screening. This part has a large demand, and the success rate of organoids for drug development has been greatly improved from the actual operation. In drug discovery, the accuracy of organoids is more than 80%.
At present, large multinational pharmaceutical companies in China have begun to purchase relevant instruments and equipment to build organoid laboratories and compare the feasibility of using organoids for drug screening. Top international pharmaceutical companies have some capacity to build organoid models, but most pharmaceutical companies will outsource organoid culture to organoid companies due to the complexity of organoid culture and their own human resource constraints.
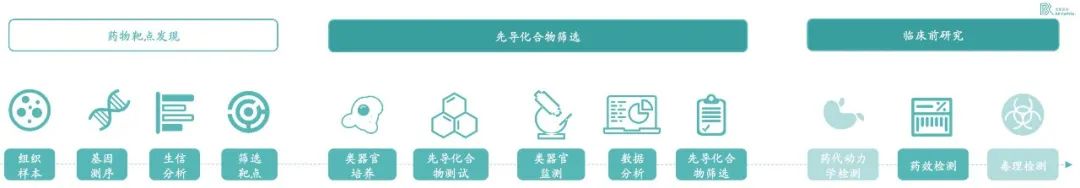
* Exhibit 4. Organoids are mainly used for drug screening and testing * Source:百榕资本专家访谈,以及分析整理
2. Expenditure on organoids for drug screening
In the research and development of new drugs, the price of organoids for drug screening is similar to that of cell lines, and the cost of single compound screening is about 500~2000 yuan. If the traditional method of drug screening is used, the drug company will conduct more rounds of drug screening due to the low clinical relevance of cell line models and animal models, resulting in a significant increase in costs; The high clinical relevance of organoids improves the accuracy of drug screening, reduces the number of cycles, significantly reduces research and development costs, and saves research and development cycles。
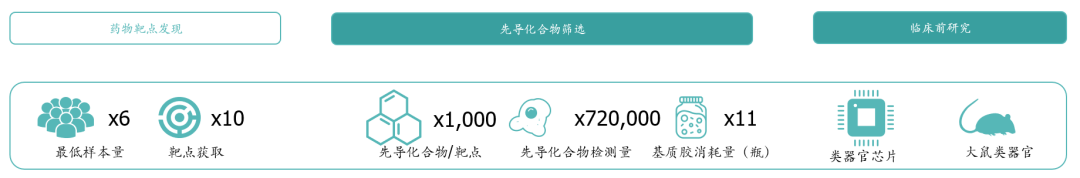
* Exhibit 5. The sample size and reagent consumption required for drug high-throughput screening are mainly used in organoids
3. Equipment consumables for organoid culture
In terms of organoid culture equipment and consumables, there are no mature commercial products in the market at present, and downstream pharmaceutical companies need to purchase customized automatic culture equipment for large-scale cultivation of organoids. In addition, it also needs a system for high-throughput analysis of organoids, and the purchase cost of a complete set of equipment is nearly 10 million yuan.
In organoid-based drug screens, the main consumables are medium, matrix glue and growth factor. In the preclinical research part, there are also pharmaceutical companies that cultivate rat organoids for efficacy testing to reduce the use of animal models.
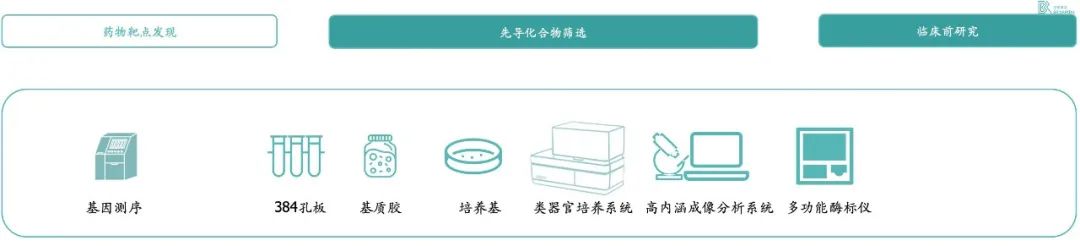
* Exhibit 6. 类器官在药物研发中涉及耗材和设备 * Source:百榕资本专家访谈,以及分析整理
L: Compared with experimental rats, organoids are simpler to maintain, and there is no need to build an animal house or clean it every day, eliminating this part of the expenditure.
2.1.3 Organoids are highly clinical and cost-effective, boosting drug research and development
Organoids can improve the efficiency of drug development, especially in tumors and rare diseases can provide disease models for drug development. Compared with traditional 2D cell lines and PDX models, organoids have higher clinical relevance and significant cost advantages over PDX models. Organoids can be prepared by collecting tumor cell samples at low cost and high throughput in vitro, which solves the pain point of low success rate of traditional models to a certain extent. In addition, there are currently more than 7,000 rare diseases, and 95% of rare diseases have no effective models to study, the emergence of organoids is expected to create effective analytical models for these rare diseases, and help related drug development.* Source:Hesperos Inc prospectus
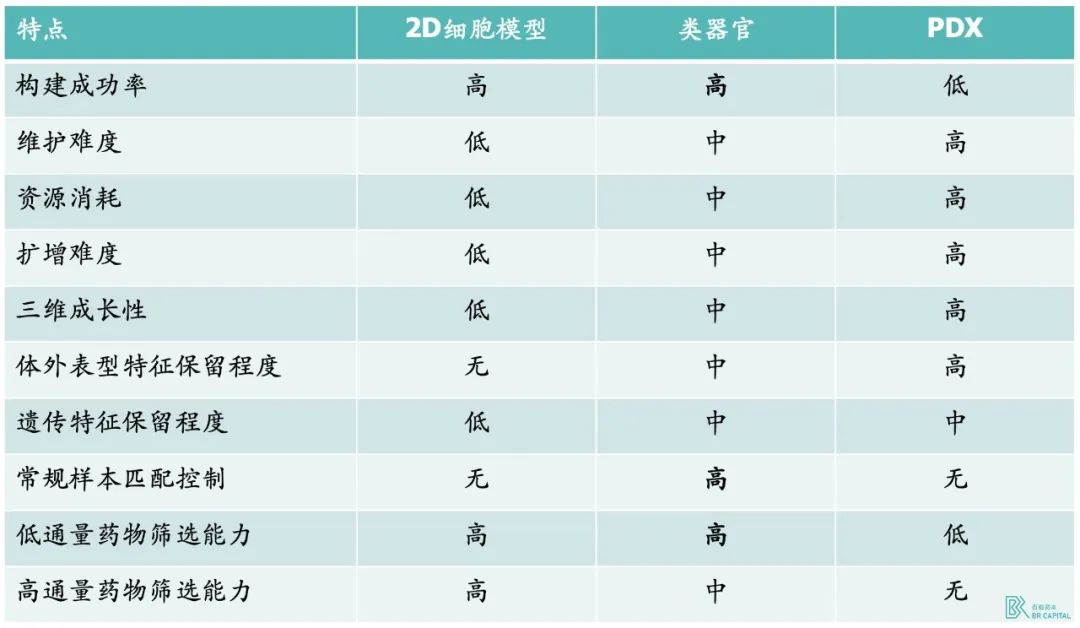
* Exhibit 7. Comparison of preclinical tumor models
In general, 2D cell lines have high efficiency and low cost, but their clinical relevance is far less than that of organoids. According to expert L, a cell line costing 5 yuan can screen 8,000 drugs, and the cost is less than 10% of that of organoids. PDX (human tumor xenotransplantation model) is less successful and expensive than organoids.* Source: 《Organoids in cancer research》
2.1.4 The maintenance cost of organoids is low, and the efficiency of drug research and development is far higher than that of traditional animal models
The huge capital and time cost combined with less than 10% of the success rate of research and development has greatly hindered the progress of drug research and development. Organoids are expected to significantly accelerate preclinical drug research.
First, organoids can be grown on a large scale using fully automated culture equipment. After the relevant tissue is collected from the human body, it can be cultured in vitro through 384 well plates, each well can be placed with different concentrations of drugs, and finally the optimal compound is selected by screening thousands of drugs.
Secondly, the length and cost of organoid culture are low. The overall time of organoid culture is only 2 weeks, traditional mice need at least 6 weeks to grow up, and the test monkey needs about 4 years, and the cultivation time of organoids is much lower than that of traditional animal models.
In addition, the pharmaceutical research and development track has continued to heat up in the past two years, and the number of new drug research and development pipelines has increased rapidly in the short term, so the price of test monkeys has risen by 100,000, and there is no market for the price, and the drug preclinical research and development needs to use a large number of animal models, and the supply side is seriously insufficient. In this case, organoids can be used as substitutes for animal models. In addition to the low culture price of organoids themselves, their maintenance price is also far lower than that of test animals, which is more cost-effective.
2.2 Organoids are used for clinical drug susceptibility detection
Because organoids can be prepared efficiently and have high clinical relevance, they have the potential to provide more effective treatment options for cancer patients in the clinic. At present, a large number of patients with advanced cancer have no effective drug treatment plan, and tumor targeting drugs cannot cover all patient populations, and there are still many patients who can only be treated by chemotherapy. Organoids can help patients to test multiple drug treatment regimens, and ultimately choose a more appropriate treatment plan to benefit patients.
2.2.1 There are fewer beneficiaries of traditional tumor concomitant diagnosis, and more general and effective ways are needed to serve patients
According to the data from the MSKCC 10,000 people study, only 36% of patients can benefit from gene sequencing, and this precision diagnosis and treatment method cannot cover chemotherapy patients.
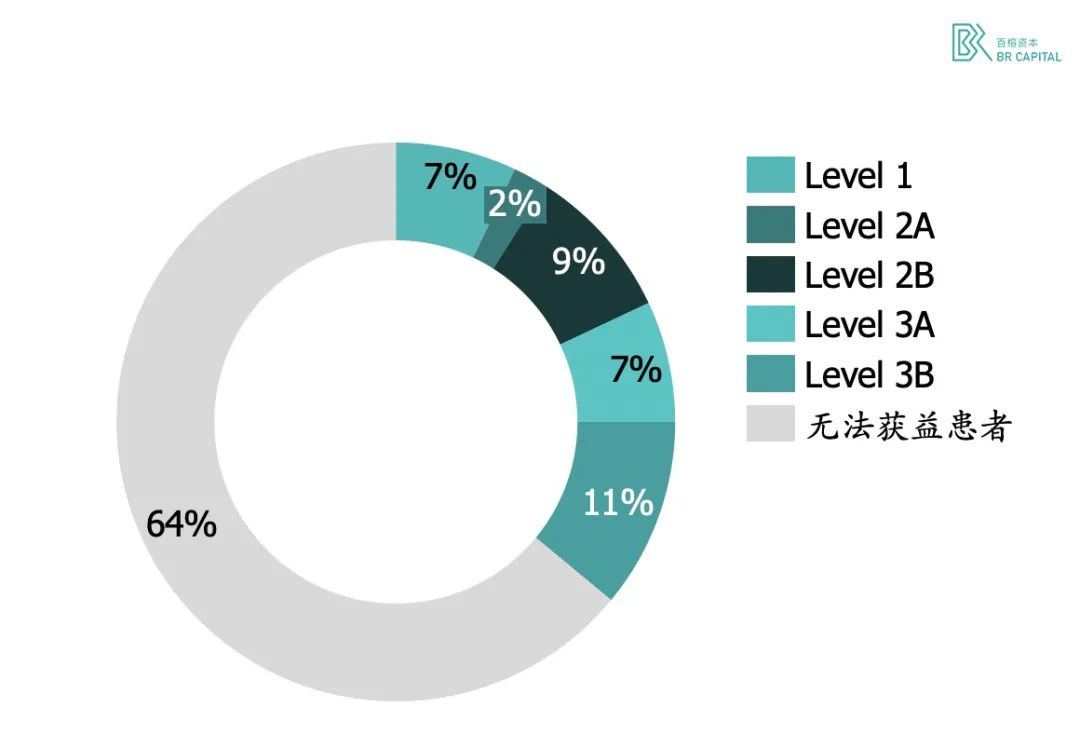
* Exhibit 8. Memorial Sloan-Kettering Cancer Center large-scale clinical sequencing analysis
Memorial Sloan-Kettering Cancer Center, the largest and oldest private cancer center in the world, in 2017 released a large-scale clinical sequencing trial that enrolled more than 10,000 cancer patients. The data showed that all mutations were divided into four levels according to clinical operability, with grade 1 to grade 3 evidence representing at least one treatable gene mutation in the tumor tissue of the patient, and the proportion of this part of the patient was 36.7%.* Source: 《Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients》
2.2.2 Organoids cover tumors with a wide variety of drugs, which are highly clinically relevant and have more advantages
In terms of precision treatment of tumors, organoid models have the advantages of low cost and high efficiency. Organoids can cultivate a large number of organoids in a very short time through high-throughput drug screening, and screen tumor drugs of different concentrations and types, whether chemotherapy drugs or targeted drugs, and try to select better drugs for targeted treatment of patients through this method. At present, ToC organoid enterprises mainly conduct sample collection and drug screening services through LDT, which is similar to the early development of NGS in China. In clinical application, the main is to conduct clinical research with the hospital.
03 Organoid enterprise development
In recent years, the commercialization of organoids at home and abroad has advanced by leaps and bounds, whether from financing cases and financing scale, as well as the practice of first-line drug companies for drug research and development, or the emergence of key policies and milestone events, all promote the rapid development of organoids.
3.1 Introduction of domestic and foreign organoid enterprises and financing
Organoids related track in the primary market in recent years, the financing news is frequent, and the financing scale continues to grow.
Overseas primary market financing situation
Since 2013, a large number of overseas startups have flooded into the organoid circuit, such as Xilis, System 1 Biosciences, KNOWN MEDICINE, CYPRE, and so on.
Xilis is the most notable of these. The company raised $70 million in funding in 2021, not only because Hans Clever, the godfather of the organoid field, joined the company, but also because Xilis's products really have huge potential: Xilis is currently developing a micro-organoid technology that offers 30 times the speed, 50 times the throughput, and 300 times the cost of traditional drug discovery methods.* Source: 《Organoids and Commercialization》
In addition, System 1 Biosciences has raised $25 million in Series A funding from Charles River Ventures and Pfizer Ventures, and the company is leveraging human brain modeling, biology and machine learning technologies. Improve the research and development ability of neurological drugs, and strengthen the research of brain diseases.
KNOWN MEDICINE, which has raised a total of $2.4 million from Khosla, Cota Capital and Y-Combinator, provides cutting-edge biological research and the latest artificial intelligence technology to help oncologists select the best drugs for patients.
CYPRE is developing a tumor modeling platform aimed at conducting 3D cell research and clinical testing on cancer patients, with seed funding from companies such as Hemi Ventures.
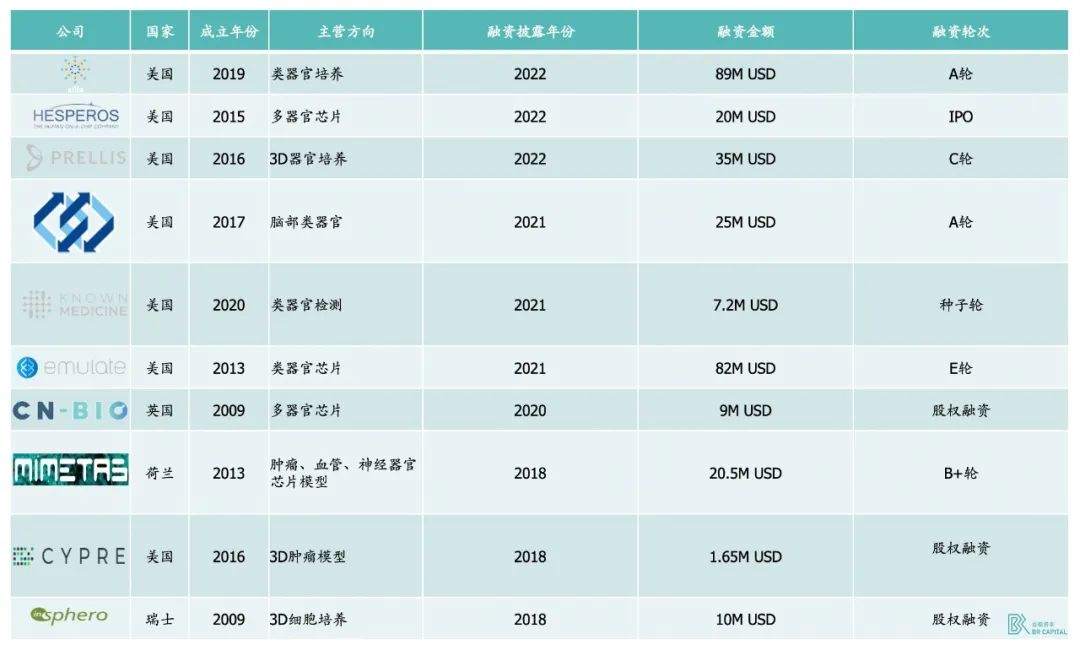
* Exhibit 9. Financing situation of overseas organoids in recent years(Xilis has raised the total amount for the A and A+ rounds)
Domestic institutions began to intensively lay out organoids
Domestic organoid participants emerged on a large scale from around 2018, and the domestic organoid industry accelerated its development.
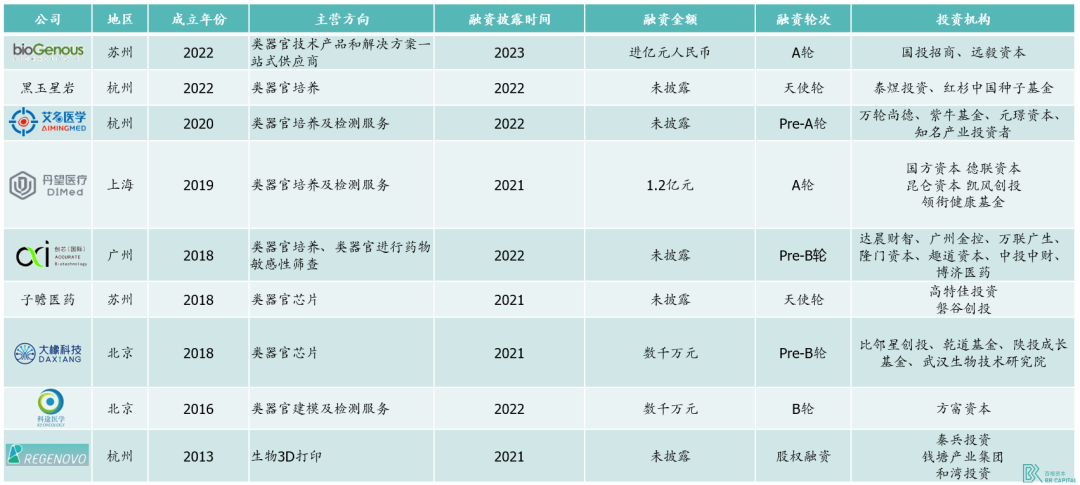
* Exhibit 10. Financing situation of domestic organoid companies in recent years (sorted in reverse order by date of establishment)
3.2 Pharmaceutical giants in the field of organoids forward-looking layout
After 2015, more and more large pharmaceutical companies entered the field of organoids through the purchase of products, cooperation authorization and investment, and carried out a forward-looking layout.

* Exhibit 11. Global well-known pharmaceutical companies in the field of organoids layout and cooperation
In addition, more than 20 pharmaceutical giants such as AbbVie, Merck, and Novartis jointly established a non-profit organization IQ Alliance (Innovation and Quality Consortium), which is committed to promoting the standardized application of organoid chips to accelerate the drug research and development process. A series of industry standards have been published for liver, kidney, lung and other models.
Domestic pharmaceutical companies Beigene and Hengrui Medical are also in the field of organoids layout.
Beigene is the only Chinese pharmaceutical company in the IQ Alliance, and in December 2021, it signed a strategic cooperation agreement with Chuangxin International to jointly establish a technology platform for the research and development of new organoids.
The translational medicine department of Hengrui Medicine has established in vitro organoid culture, which can conduct drug research in simulated human organs and tumor microenvironments;
R&d Director of internationally renowned pharmaceutical companies L: At present, the top 30 pharmaceutical companies have been using organoids in the process of drug research and development. We have laid out the organoids since 2015, and started to recruit organoids experts to build internal organoid research and development departments since 2019. At present, 7 or 8 laboratories use organoids to help internal drug research and development. More than 100 people have been recruited so far.
In addition, according to the public recruitment information of major CRO and pharmaceutical companies, in recent years, pharmaceutical companies have actively published the recruitment of organoid researchers JD information and established their own organoid research and development departments, such as Fosun Pharmaceutical, Medisi, Pharmaron and so on.
3.3 Policies and guidelines continue to be issued
Favorable policies and guidelines on organoids at home and abroad continue to be released. In foreign countries, Europe and the United States have been advocating to reduce the use of animal models, while the cost of obtaining human tissue samples is higher than that in China, so when the US Senate passed the FDA Modernization Act in September 2022 to promote the reduction of the application of preclinical trials on animals, it received a lot of attention; In addition, the use of organoids in rare disease disease models has also allowed more patients to see the dawn of recovery.
Domestically, the state continues to promote the development of stem cell-related technologies, and in early 2021, organoids are listed as the first batch of key special tasks in the "14th Five-Year Plan" national key research and development Plan, and at the end of 2021, organoids are listed for the first time in the verification guidelines for gene therapy and cell therapy non-clinical pharmacological studies of gene therapy products. In mid-2022, the first expert consensus on organoid guided tumor precision drug treatment was also launched, helping the development of domestic organoids in tumor precision therapy. Below is a list of major events in the field of organoids in recent years.
On January 28, 2021, the Ministry of Science and Technology issued the Notice on the Guidance for the application of six key projects in the "14th Five-Year Plan" National Key Research and Development Plan for 2021, and listed the "organoid based malignant tumor disease model" as the first batch of key special tasks in the "14th Five-Year Plan" national key research and development Plan.
In November 2021, China CDE included organoids in the validation guidelines for gene therapy and cell therapy for the first time in non-clinical pharmacological studies of gene therapy products.
In July 2022, China's first expert consensus on organoid guided tumor precision drug treatment was published.
In August 2022, for the first time, the US FDA approved an investigational therapy by Sanofi to enter clinical trials based solely on preclinical efficacy data obtained in human organ-on-a-chip studies, combined with existing safety data. Instead of using efficacy data from traditional animal studies, this pipeline preclinical study used efficacy data from organoon-on-a-chip models that mimic two rare autoimmune demyelinating diseases that currently lack animal models that effectively mimic disease symptoms and therefore cannot be used to evaluate the efficacy of potential therapies. The application of organoids in rare diseases has also allowed more patients to see the dawn of recovery.
In September 2022, the U.S. Senate passed the FDA Modernization Act, which aims to reduce the use of preclinical testing on animals and replace it with more modern scientific methods. This groundbreaking legislation has the potential to reduce the use of millions of animals in the coming years and provide safer and more effective medicines for patients. At present, organoids seem to be the most likely technology to partially replace animal testing.
3.4 Demand for organoids related services and products continues to emerge
Universities and medical terminals have a large number of organoid culture needs, procurement equipment from overseas enterprises, in urgent need of domestic mature solutions; After the release of the first expert consensus on domestic organoids in 2022, the number of tenders for related projects has soared.
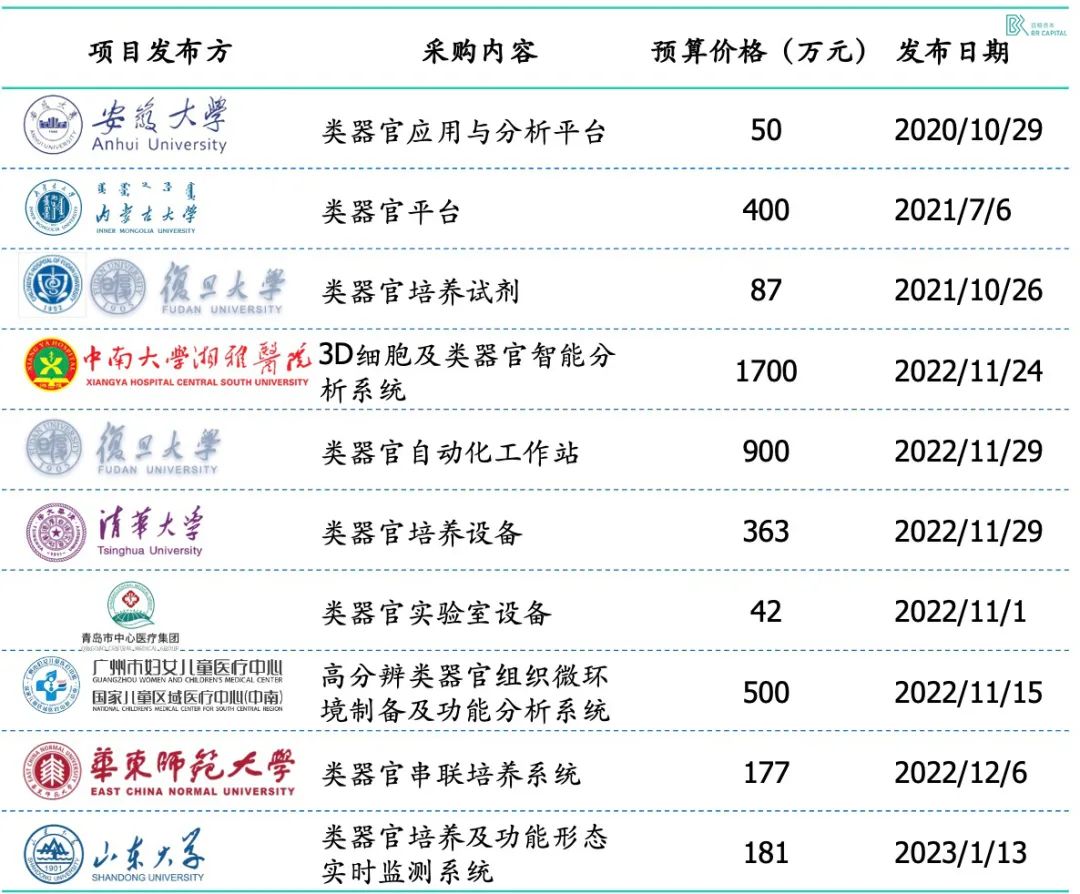
* Exhibit 12. Domestic organoids related services and equipment consumables bidding information
04 Problems encountered in the commercialization of organoids and potential solutions
In the future, organoids will develop in the direction of process standardization, model complexity, culture and screening automation. At present, the development of organoids in China mainly focuses on the following two aspects: regulations and standards, and organoid culture technology.
4.1 Regulations and standards
According to the commercial scenario of organoids, there are three main problems in the commercialization of organoids.
Problem 1: There are no authoritative guidelines for the provision of drug susceptibility tests for ToC end organoids, and it is difficult for doctors to use organoid tests in clinical practice
Solution direction: Major pharmaceutical companies, scientific research institutions and organoid enterprises are conducting clinical research to promote the application of organoids in clinical practice.
First of all, at the ToC end, the main application of organoids is clinical drug sensitivity detection, but at present, there is no authoritative and accurate guide for the use of organoids in clinical cancer patients in the world to provide a basis for doctors' decision-making.
Therefore, the current clinical application of organoids is still in the early trial stage, and all parties are actively promoting the clinical application of organoids.
For example, two expert consensuses on guiding organoids for precise treatment of clinical tumors were released in China in mid-2022, in which the potential of organoids for clinical application was recognized, and the types of cancers and the range of cancer patients suitable for trying organoids technology were given. In addition, there are also hospitals in clinical research with large pharmaceutical companies and organoid companies, as of September 2020, there have been 63 clinical trials in the FDA official record. Domestic organoid clinical trial studies registered in 2017 and approved by the Ethics Committee also cover multiple cancer types.
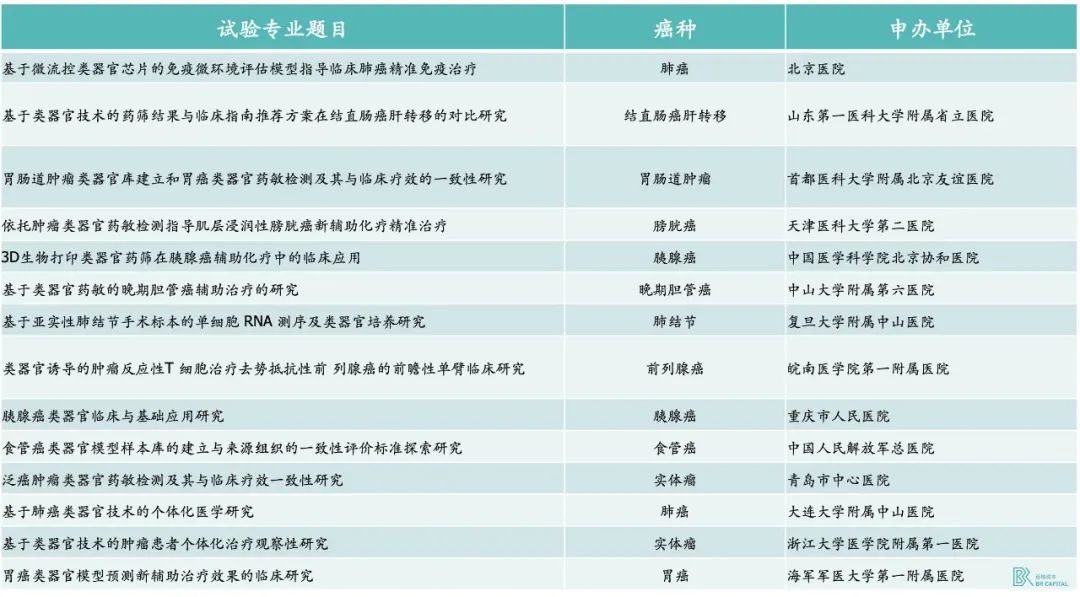
* Exhibit 13.Newly registered organoids related clinical trials in China in 2022 * Source: China Clinical Trial Registry
Problem 2: TOB-end drug companies using organoids for drug screening are difficult to directly use test data to submit for registration
Solution: Pharmaceutical companies use organoids as additional tests in the drug development process
At the ToB end, because drug review institutions do not have uniform evaluation criteria for drug test data from organoids, at present, organoids are mostly additional experiments for drug research and development, and corresponding data cannot be directly submitted to the review department to help drugs pass IND.
Despite the above problems, pharmaceutical companies are still actively using organoids for drug research and development, because the use of organoids can greatly improve the efficiency of drug research and development.
L: The precision of organoids for drug screening is as high as 80%, which greatly improves the success rate of drug research and development, and we are more inclined to choose organoids for drug research and development. The current success rate of developing drugs with mice is very low and can be very misleading. Cost is only one factor to evaluate the quality of technology, for example, even if the cost of organoids is 10 times higher than that of mice, but the success rate of drug development of organoids is as high as 80%, and mice can not produce a drug, then we will use organoids, because the direction of drug development with mice is the opposite.
Problem 3: organoid culture lacks training norms, and the cultivation methods of various organoid enterprises are not uniform, which is difficult for pharmaceutical companies to apply
Solution direction: Pharmaceutical companies develop organoid reception standards, and organoid companies provide products that meet the conditions
In the process of organoid culture, there are a lot of non-standardized links to standardize the culture and analysis of organoids. For example, the details of the process such as the size of organoid culture, the selection of cell sources used for tumor organoid culture, and the types of animal serum or conditioned medium used for organoid culture have yet to be standardized.
Therefore, whether the pharmaceutical or organoid companies have a clear solution is the key to the commercialization of organoids in the B end. According to the interview, at present, large pharmaceutical companies have their own specifications and requirements for organoid products, and they will not interfere with the specific organoid culture methods of organoid companies. As long as the organoid products cultivated by the latter can ultimately meet the standards and relevant parameters of pharmaceutical companies, there is a willingness to pay.
L: When we choose organoid suppliers, we have high requirements for batch stability of organoids. Organoids can generally be transmitted 40-50 generations, and the trend of testing drugs between batches is unchangeable; Organoids are between 100-300 microns in size; Secondly, we need to ensure that organoids can not be contaminated; For different models, quantitative indicators are different. At present, some enterprises in the industry do a good job of publicity, but do not actually do it; L Generally go to the laboratory to see the growth of organoids in person, and roughly judge the technical strength of the company through naked eye observation.
For the commercialization of organoids in ToB, we also further communicated with expert L to understand the demand of pharmaceutical companies for organoids. As the experts said, organoid enterprises have intellectual property rights and rich experience, and pharmaceutical companies do not have cost advantages for training themselves, so pharmaceutical companies are more willing to outsource this part of their business.
L: Pharmaceutical companies themselves can carry out organoid culture, but manpower is limited, and pharmaceutical companies themselves are reluctant to support relevant personnel; In addition, organoid suppliers have intellectual property rights and rich testing experience, and pharmaceutical companies can also do it themselves, but they are not skilled enough, so the current organoid culture service price is acceptable for pharmaceutical companies.
4.2 Organoid culture technology
In terms of organoid culture technology, there are also some problems, including the inability of tumor organoids to fully restore the tumor microenvironment, the lack of equipment for automated high-throughput organoid culture, and the data difference between primary and pass cell culture organoids before and after testing. However, these problems are more promising than the obstacles in the regulation and cultivation standards of organoids, and potential solutions will appear in the short term.
Problem 1: There is currently no commercial equipment dedicated to fully automated high-throughput culture and analysis of organoids worldwide
Solution direction: At present, there have been a number of companies to overcome related equipment
At present, there is no equipment dedicated to the automatic culture of organoids in the world. At present, large pharmaceutical companies will buy pipette workstations and customize and develop automatic liquid culture modules for organoids from related companies, and the overall customization and development cost is high, with the price of one device exceeding 5 million yuan; In addition, such equipment can not include other functional modules of subsequent organoid culture, high-throughput screening, and analysis, so the demand for integrated equipment with complete functional modules of the whole process will usher in an outbreak in the future. There are already a number of companies in China that have claimed to have related equipment in research, and we will see related products on the market in the short term.
Problem 2: The data for drug testing of primary cells and passing cells may be quite different
Solution: Test only with passing cells
L: We generally use pass-through organoids and do not use primary cell tests because it would be troublesome to get them from patients every time. The organoids used by the drug screen are all subgenerations, generally used after 3 generations; Although there are different generations, the trend of drug efficacy in different generations is the same; However, there is no fixed standard at present, and the available generation of each organoid is different. We will explore each organoid, and we can develop new standards when more data is accumulated.
Problem 3: Organoids cannot completely restore the internal environment, especially the tumor microenvironment
Solution direction: Research on multicellular co-culture technology or cooperate with organ chip to restore the internal environment as much as possible
At present, organoids cannot fully construct the internal environment. Organoids lack nerve cells, interstitial tissue, and elements of the immune system, as well as blood vessels, all of which are critical in tumor development. For example, some drugs do not target the tumor, but kill the endothelial cells inside the tumor, reducing the energy supply to the tumor, and the current organoids can not simulate this situation. The current solution is to model tissue function in vivo through organochips; In the aspect of tumor organoid culture, multi-cell co-culture environment such as tumor organoids, tumor fibroblasts and immune cells is carried out to restore the environment in the body to the greatest extent.
4.3 Differences in organoid participants and future commercialization
At present, there are nearly a hundred domestic companies specializing in organoids and companies extending organoid services from stem cell and CRO companies, so a question naturally emerges: What kind of companies can stand out in the future?
We believe that the services and products provided by organoid enterprises can be divided into three levels, and the enterprises will gradually develop a higher level of business development and growth:
Level 1:Organoid culture and analysis services for hospitals or pharmaceutical companies (drug sensitivity testing)
Level 2:For pharmaceutical companies, CRO, research institutes, hospitals and other institutions to provide automatic organoid culture equipment and consumables
Level 3:We provide standardized and customized organoid chips as disease models for drug screening and testing for pharmaceutical companies and research institutions
4.3.1 Level 1:Organoid culture technology is the foundation
Organoid culture service and technology is the basic ability of related enterprises, and this part has the largest potential market space, but the excessive period cost has a certain inhibition on the profitability of the company. Although the clinical drug sensitivity test of organoids is provided for patients, the key steps of the popularization and recommendation of organoid services, sample collection, sample delivery, ethics, etc. are coordinated by the hospital. Of course, it cannot be denied that some patients voluntarily learn about the public information about organoids and take the initiative to try them. Therefore, the current mode of ToC in organoids is more appropriate to be represented by ToH (Hospital).
As a business model of ToH, with reference to the development of NGS in China, organoid culture service providers are more likely to fall into a situation similar to that of NGS service providers in the ToH market. That is, the homogenization of each service is serious and the market competition is fierce. If we can master the R&D and supply capabilities of upstream equipment and consumables like Illumina or BDA Intelligent Manufacturing, organoid enterprises can further enter Level 2.
4.3.2 Level 2:The barriers are high and the needs are urgent
In terms of equipment, whether it is ToC or ToB's organoid culture service, automatic high-throughput organoid culture is a necessary condition. As mentioned above, there are currently no listed equipment dedicated to organoid culture in the industry, and various companies are developing it. However, only the culture of organoids is not enough, and it is difficult to observe the morphology of organoids of 100μm size with the naked eye. Therefore, the follow-up automatic high-throughput analysis equipment of organoids is also one of the necessary equipment, such as high-connotation confocal microscopy. If further discussed, the traditional cell culture plate is suitable for 2D cell culture, while the organoid is a three-dimensional tissue, so the traditional culture plate is not fully suitable for 3D scanning, and this problem needs to be solved for more accurate observation. To sum up, organoid culture equipment is a mechanical, engineering, and optical cross-disciplinary composite product, the company team has high requirements, simple medical team is difficult to develop such products, so the current market is showing a lack of supply.
In terms of consumables, the main reagents used in organoids are matrix glue and medium, and the cost of single pore organoids culture is about 15% of matrix glue and 45% of medium. At present, Corning's Matrigel matrix adhesive occupies more than half of the global market share of matrix adhesive used in organoid culture, and is known for its excellent effect and excellent batch stability. The domestic price is about 4000 yuan/bottle (1 bottle 10ml). Due to the potential of the matrix gum market and the instability of Matrigel matrix gum as an animal source, companies are also developing their own matrix gum or hydrogel alternatives, but Corning's position is still unchallenged at this stage.
We believe that companies that make organoid culture equipment and consumables are now more certain in the market growth; And because such products are not low technical threshold, once the enterprise successfully breaks through, it will have a significant competitive advantage, which is difficult to be easily replaced, and the role of the seller can have stronger stability and high gross profit characteristics.
4.3.3 Level 3:Long-term development will be better
We believe that organoid chips are the products with the highest technical barriers and the broadest application prospects in the field of organoids, and the corresponding enterprises have greater room for growth in the future. In terms of technology, the organoid chip is a synthesis of organoid + microfluidic chip technology, which has the highest requirements for the core team of the enterprise, which must have both biological and medical background, but also have composite talents with engineering and mechanical backgrounds, such a team or expert can not be sought; In terms of application, compared with traditional animal models and cell models, organochips can create more diversified disease models to break barriers for new drug research and development, especially after FDA approved drugs from Hesperos organ chip preclinical experimental data into IND event in 2022. Make people more optimistic about the future of organ chips.
However, the current pain points of organoid chips are also relatively obvious, mainly because the price is too high, the price of a single piece is several hundred yuan, there are certain barriers to large-scale application downstream, and commercialization is still in the embryonic stage. According to Hesperos' prospectus, the company's 2021 revenue is $5.2 million, of which $4.36 million is a grant from the National Institutes of Health, and only $830,000 is commercial revenue.
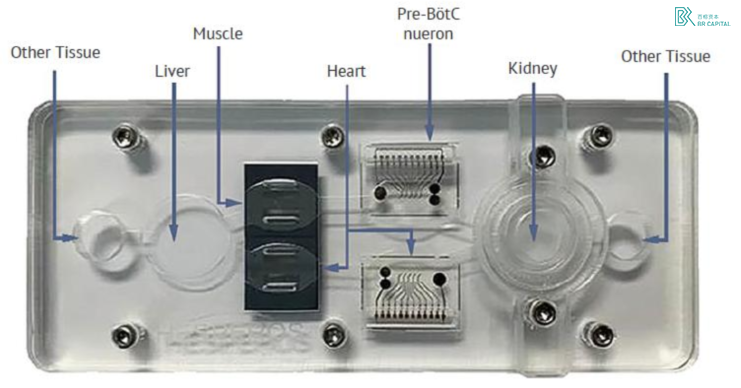
* Exhibit 14. Hesperos Multi-organ Chip * Source: Hesperos prospectus