
Source: Aoyin Capital, Weidu
Organoid technology, which uses stem cells to directly induce 3D tissue models, offers a powerful method for human biological research.
Stem cell research has become an important branch of biological research, and one of the purposes of studying stem cells is to help us better understand the various dimensions of life. Cells and cells are connected together through the interstitium to form cell clusters, and various organs and tissues are formed due to the differential expression of genes in cells.
Scientists have long used animal models for disease research and drug development. With the development of stem cell technology, scientists can use new culture methods to induce pluripotent stem cells and/or adult stem cells to produce some three-dimensional structures that resemble tissues or organs in the body - and organoids are born.
1. Science popularization and market size
Organoids are tissue analogues with certain spatial structure formed by three-dimensional (3D) culture of adult stem cells or pluripotent stem cells in vitro. Although organoids are not human organs in the true sense, they can simulate real organs in structure and function, and can maximize the structure and function of tissues in the body and can be long-term stable subculture (therefore, they are also known as "micro organs").
The development of organoids has been hailed as one of the most exciting advances in stem cell research over the past decade. The term "organoid" was proposed as far back as the 1980s, but it wasn't until 2009, when Dutch scientist Hans Clevers' team successfully cultured Lgr5+ intestinal stem cells in vitro into three-dimensional structures with crypto-like and villous epithelial regions. The study of small-intestinal organoids opened a new chapter in the rapid development of organoids [Nature.459, 7244:262-265].

Figure 1. Development history of organoids
In 2013, organoids were named the top 10 technologies of the Year by Science. In early 2018, organoids were named the Best Method of 2017 by Nature Method. At present, a variety of organ organoids have been successfully constructed, including small intestine, stomach, colon, lung, bladder, brain, liver, pancreas, kidney, ovary, esophagus, heart, etc., including not only normal organ tissue organoids, but also corresponding tumor tissue organoids.

Figure 2. Topography of different organoids: (a) Small intestine topography; (b) colon topography; (c) esophagus topography; (d) Stomach topography. (e) Liver organoid topography. (f) Pancreas organoid topography. (g) Lung organoid topography. (h) Breast organoid topography. (i) Kidney organoid topography. (j) Brain organoid topography
In recent years, a search for "Organoids" in PubMed published literature shows a sharp increase in the number of literatures related to organoid technology, many of which are in top journals such as CNS. China's ranking in the number of organoids published in the world jumped from sixth (2009-2019) to second (2020), second only to the United States. The improvement of China's scientific research accumulation will accelerate the process of organoid industrialization.
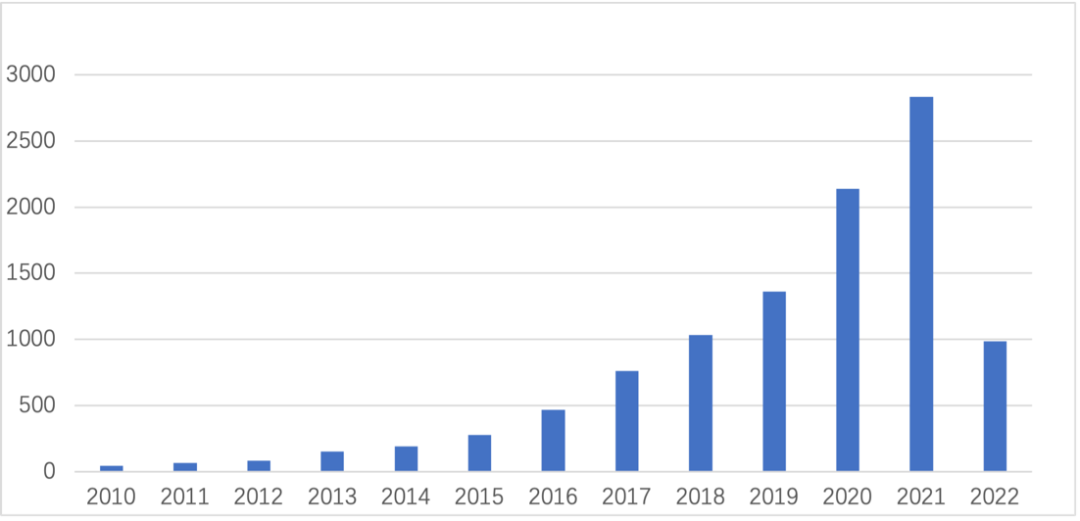
Figure 3. Number of published organ literatures
Organoids can be derived from adult stem cells (ASCs), pluripotent stem cells (PSCs)(embryonic stem cells, ESCs), and induced pluripotent stem cells (iPSCs). The organoid culture system mainly consists of matrix glue, factors required for maintaining organoid ecology and factors required for differentiation. The matrix glue contains collagen, nestin, fibronectin, etc., which provides the matrix for organoids to form three-dimensional spatial structure. The main purpose of maintaining organoid ecological factors is to promote cell proliferation and inhibit cell apoptosis. The commonly used matrix adhesive is Matrigel® of BD Biosciences in the United States, which is in a relatively monopolistic position in the industry and has a high price. Matrigel can produce bioactive matrix materials similar to the basement membrane of mammalian cells, helping many types of cells reach attachment and differentiation.

Figure 4. Methods of obtaining organoids [Organoids: Definition, culturing methods, and clinical applications. CytoSMART. 2022]
As a tool, organoid technology has broad application prospects in basic research and clinical research, including developmental biology, disease pathology, cell biology, precision medicine, and drug toxicity and efficacy testing. The technology also offers great potential for regenerative medicine, opening up the possibility of autologous or allogeneic cell therapy by replacing damaged or diseased tissue with organoid cultures.
The main development direction of organoid technology is to apply organoid technology to clinic, guide clinical medication and precision treatment. In fact, organoid technology has been included in clinical trials since 2016, and 63 clinical trials have been officially filed with the FDA as of September 2020. Since 2017, there have been 20 organoid clinical trial studies registered in China and approved by the Ethics Committee, covering 8 cancer types, mainly focusing on the efficacy prediction of chemotherapy methods, but existing studies have begun to focus on the application of immunotherapy in organoids (Changhai Hospital, PD-1). From the perspective of cancer distribution, most of the cancer types studied in China are digestive system tumors, pancreatic tumors and breast tumors.

Figure 5. Cancer species with ≥3 organoid clinical trials conducted since 2017
According to related reports, the North American organoid market reached $291.39 million in 2019 and is expected to reach $1,466.47 million in 2027, which will grow at a CAGR of 21.7%. According to the International Agency for Research on Cancer (IARC) of the World Health Organization, there will be 19.29 million new cancer cases globally in 2020, and 4.57 million new cancer cases in China. In 2020, there will be 9.96 million cancer deaths globally, and 3 million cancer deaths in China. It is estimated that the domestic organoid market will reach more than 10 billion, with the continuous emergence of new drug pipelines, clinical and patient demand for personalized treatment is increasing, the market space will continue to grow.
2. Comparison of organoids with other models
Currently, the most common models are cell lines, yeast, caenorhabditis elegans, Drosophila melanogaster, common mice, zebrafish, and patientderived xenograft (PDX), which have been widely used to study cell signaling pathways, identify potential drug targets, and develop new drugs. But it also revealed many limitations.
(1) 2D (Two-dimensional) cell lines are easy to operate, but usually contain only a single cell type, such as tumor cell lines, lack the heterogeneity of the original tumor and are genomically unstable in vitro culture.
(2) The mouse model is the most commonly used research model in most laboratories, but it is inherently different from human specific biological phenomena, such as brain development, metabolism, and drug efficacy.
(3) Although Non-human primates (NHP) are closest to humans in the phylogenetic tree and possess highly similar immune systems, brain structures, and cognitive functions, they are expensive, subject to ethical limitations, limitations in imaging observation, uncontrollable complex variables, and difficulties in high-throughput drug screening. Limit its widespread use.
Therefore, in accordance with the "Reduce, Reuse, Recycle" principles and the principles of species conservation, there is a need to explore stable, easy to operate, and widely applicable model alternative systems that can reduce differences with the human body to study genetic diversity, disease pathogenesis, and predict drug response.
Clinical trial data confirm that rectal cancer organoids (Rcos) can accurately summarize the pathophysiological and genetic changes of corresponding tumors. The chemoradiological response was highly matched with RCO response, with accuracy of 84.43%, sensitivity of 78.01% and specificity of 91.97%. These data suggest that Pdos (Patient-Derived Organoids) are clinically predictive of responses in patients with LARC and may serve as an adjunct diagnostic tool in the treatment of rectal cancer.

Figure 6. Comparison of organoid models with 2D cell lines, mouse models, and non-human primate models
As shown in the figure above, according to the characteristics of organoid models, the application of complementary rather than replacing traditional models will open up new ways for human biology research.
Compared with the traditional model, organoids have the following characteristics:
2.1 Fast Speed
The success rate of organoid construction is high and the culture speed is fast. Generally speaking, the drug screen can be performed after a week of organoid culture. The whole process from sample collection to drug sensitivity results can be well controlled within 2 weeks [NEngl J Med. 2019, 380(6):569-579].
2.2 High flux
In terms of the drug flux that can be screened, organoids can not only screen multiple drugs on the pore plate, but also test different concentrations of each drug, and multiple experiments can be carried out in parallel.
2.3 High clinical relevance
The clinical relevance and predictive effectiveness of organoids for cancer drug screening have been fully confirmed in several studies. Vlachogiannis G's team published a landmark study on in vitro drug susceptibility testing of tumor organoids to guide clinical drug use in Science. 110 tissues were extracted from 71 metastatic gastrointestinal cancers to construct organoids, and a total of 55 anticancer drugs were tested. The results of the study showed that the organoid drug screen achieved 93% specificity, 100% sensitivity, 88% positive prediction rate and 100% negative prediction rate, demonstrating high clinical relevance [Scient. 2018, 359(6378): 920 -- 926].

Figure 7. Comparison of drug sieve models
3. Organoid industry chain sorting
The upstream industry chain of organoids is mainly to provide consumables for organoid culture (including media, cell growth factors, frozen liquid, digestive fluid, matrix glue, etc.) and equipment. These include established providers of cellular reagents such as STEMCELL Technologies and emerging companies focused on providing organoid reagents such as Prellis Biologics' vascularized 3D Scaffold and X cell Biosciences' microenvironment simulation system.
In the middle reaches of the industrial chain, it mainly provides the cultivation of human or animal organoids, the frozen storage of organoids and the passage of organoids.
Downstream customers of the industrial chain are mainly divided into scientific research applications (universities/hospitals), clinical applications (hospitals/patients) and research and development applications (pharmaceutical companies /CRO). Part of human disease analysis is difficult to complete through animal model simulation, and animal model cultivation cost is high, time-consuming, low repeatability, organoid model can simulate normal tissues and tissues of different stages of cancer transformation process; And its cultivation system is simple and easy to operate, the cost of time and money is low, and has high efficiency.

Figure 8. Organoid industry chain
At present, the research and application of organoids are mainly concentrated in the direction of disease model research and curative effect prediction. At present, a number of universities and hospitals have carried out corresponding scientific research, such as the Chinese Academy of Sciences, Tsinghua University, Zhejiang University, Beijing Tiantan Hospital, Zhejiang University and so on. The advantages and potential of the PDO technical route compared with the traditional route have been recognized by the academic community. The number of academic papers containing Organoid published by PUBMED in 2019 has exceeded the number of PDX model papers published in 2019, and the number of organoid related clinical trial studies registered or approved by the Ethics Committee in China in 2017 has reached 20.
The research market of organoids is growing relatively slowly, and the research market is highly customized and needs to provide standardized services. With the further development of the scientific research market and the advancement of the guidelines, the domestic market has gradually expanded, and we believes that organoids will have unique advantages in cell and gene therapy, immunotherapy and other model scientific research services.
Clinical research applications currently mainly provide precision treatment for patients with advanced cancer. Direct drug testing by patients is time-consuming, risky and painful, especially for tumor patients who lack effective drugs and can only be treated by chemotherapy, it is difficult to find effective solutions in time. And organoids can replace patients to test drugs to achieve precise treatment. At present, organoids are mainly used for sensitivity detection of chemotherapy drugs, and they have greater potential for targeted drugs and immunotherapy in the future. At present, hospitals including Nanfang Hospital, Changhai Hospital, West China Hospital, Fudan University Affiliated Cancer Hospital and other hospitals have carried out corresponding clinical studies.
At present, the clinical market of organoids is still in the cultivation stage: due to the absence of guidelines, the awareness of patients and the willingness of clinicians to submit tests are limited. With the increase of clinical application of PDO, it is expected that the demand for PDO in the clinical market will increase significantly under the trend of precision therapy. Organoids are of great value to patients, especially those with tumors that lack effective drugs and can only be treated through chemotherapy, and can be used as an effective tool to achieve precision therapy.
The application of organoids in the commercial market is mainly in the direction of new drug research and development and expansion of indications. At present, about 85% of preclinical drugs fail to develop after entering clinical trials, resulting in huge costs and losses. However, organoids can be evaluated more fully before clinical trials, which is of great value for reducing the cost of later drug development. In the development of anti-tumor drugs, PDO can reflect tumor heterogeneity with high throughput and low cost, and effectively make up for the shortcomings of PDX animal models. Phase 0 "quasi-clinical trials" of organoids as "patient surrogated" can improve the success rate of clinical trials. Foreign large pharmaceutical companies including Roche, Eli Lilly, etc., and domestic companies including Pioneer, Hengrui, Qilu, Wuxi Apptec and other pharmaceutical companies and CRO are also involved.
At present, the organoid drug research and development market is still in the initial stage, and pharmaceutical companies are still in the wait-and-see stage, and the current revenue of organoid companies is mainly for verification services. Organoids are not a necessary option for new drug submission, pharmaceutical companies are still following the applicability strategy, and the maturity of organoids technology and the inventory of samples are still limited, which has become the main concern for decision-making. However, it is undeniable that organoid technology can greatly enable drug companies to do risk management, and reduce costs and increase efficiency, and the drug research and development market will have the greatest commercial value. In the context of global drug innovation, pharmaceutical companies have a sharp increase in demand for new drug research and development to reduce costs and increase efficiency and improve success rates, and their willingness to pay for the value brought by organoids in the future is stronger than in other markets.
4. National policies help organoids
In the past two years, the Ministry of Science and Technology, the Health Commission and the CDE have continuously issued policies to loosen the extensive application of organoids, while the supervision of human heritage resources has gradually tightened, and the organoid industry will develop in a policy environment that encourages and standardizates policies.
On January 28, 2021, the Ministry of Science and Technology issued the "Notice on the" 14th Five-Year "National Key Research and Development Plan of six key special projects for 2021 annual project declaration Guidelines", which listed the "organoid based malignant tumor disease model" as the first batch of key special tasks in the "14th Five-Year" national key research and development plan.
On November 30, 2021, the Drug Evaluation Center of the State Drug Administration issued the "Guiding Principles for Non-clinical Research and Evaluation of Gene Therapy Products (Trial)" and the "Guiding Principles for Non-clinical Research of Gene Modified cell therapy Products (Trial)" (1), which for the first time included organoids in the guiding principles for gene therapy and genetically modified cell therapy products.
In the clinical market, the state encourages the implementation of LDT and ICL, and promotes the transformation of scientific research results into clinical applications. Hospitals can develop innovative IVD reagents according to clinical needs and use them in hospitals. Among them, hospitals in Shanghai Pudong New Area can carry out LDT first. Shanghai Municipal Health Commission promotes the implementation plan to encourage LDT and third-party medical examination institutes, supports municipal medical and health institutions to take the lead in establishing scientific research results transformation institutions, and encourages and supports medical and health institutions to entrust third-party service institutions to carry out technology transfer services.
5. types of organ industry development direction
At present, there are three main focuses of organoid technology development, namely organ chip, AI high-throughput automation, and organoid sample library (Biobank). The engineering solution based on microfluidic and 3D printing technology will solve the existing drawbacks of organoids, and realize the transition from the research and development end to the commercial application end, and become a standardized application tool. AI high-throughput automation can be applied to sample quality control and standardization of the culture and use process, improve the success rate, optimize and save the time of manual participation, and facilitate clinical application. The establishment of Biobank makes it possible to screen physiologically related drugs, which is conducive to translating scientific research results into market applications.
5.1 Microfluidic chip
Compared with other technologies, microfluidic chips and 3D bioprinting solve the problems of difficult material molding, short modeling and molding time, and small sampling, and the larger volume can meet the needs of drug transport dynamics.
Compared with traditional animal experiments, microfluidic chips have three technical advantages:
(1) More cost effective: Organs on microfluidic chips are more cost effective than traditional animal tests, and can measure more indicators with smaller cells/tissues than traditional organoid culture tests.
(2) Better simulation of the environment and reaction in vivo: able to control cells and specific tissue structures, and with tissue vascularization and perfusion capabilities.
(3) Easy monitoring of health status and dynamics: Incorporate real-time tissue function sensors, such as microelectrodes or optical microscope markers (such as fluorescent biomarkers).
Microfluidic chips are currently mainly used in scientific research scenarios, and still face technical challenges, the main challenges lie in three aspects:
(1) Integration technical difficulties: scientific research: domestic research in the field of multi-purpose film, but the processing cost is high, many school research institutions in the film integration, but do not do well; Commercial field: Most of the petri dish/petri dish structure is completed with water flow and pressure, the technical difficulty of membrane structure is greater than the integration of membrane and membrane processing technology, petri dish as a complete set of systems, integration is more difficult.
(2) Low repeatability: the regulation of the dose concentration and the collection of the final sample, not every experiment can be repeated well. The cost performance is not high.
(3) Hardware barriers: The gap with foreign countries mainly lies in the accuracy and durability of the lithography machine.
5.2 AI combined with high-throughput automation
Similar to other tracks, AI in the field of organoids is more about solving mechanizable artificial problems in a more convenient way in the future large-scale promotion and clinical use. Current AI research hotspots pay more attention to the organoid culture end, and the combination of the use end and big data will bring more disruptive business opportunities. In the future, the intelligent solution that combines AI and automation technology with microfluidic chips to form hardware and software integration will become the mainstream product form of commercialization in the future.

Figure 9. Hot spots of AI research
5.3 Biobank
At present, Biobank is still the only legal source of samples from hospitals, and several institutions have begun the construction of sample banks. With the continuous strengthening of supervision by the Human Heritage Office of the Ministry of Science and Technology, Biobank will have more government participation and supervision in the future.

Figure 10. Biobank industry chain
The current difficulties of Biobank are as follows:
(1) The tissue in the sample bank is limited, and the current number of organoid models and the cancers covered are far less than PDX:
① The main storage for the mainstream cancer: lung cancer, bowel cancer, stomach cancer, breast cancer, in addition to more pancreatic cancer and head and neck cancer.
② Because organoid companies mainly obtain samples by providing drug sensitivity tests, the storage capacity of normal tissue organoids is very limited.
(2) The cost of organoid model cultivation and maintenance is high, and the technology is also insufficient. The failure rate of resuscitation and expansion of organoids is high, and the stability of frozen storage needs to be further explored.
6. types of organ industry competition pattern
The organoid industry has developed rapidly in European countries, which is closely related to the earliest start and accumulation of organoid scientific research in Europe. Hubrecht Organoid Technology (HUB), founded by Hans Clevers, the leader of organoids, is the earliest research and development center for organoids. HUB technology licensing has led to the emergence of a number of organoid companies, including Epistem, Cellesce, Crown Biosciences, STEMCELL Technologies, and others.
In the field of organoids, China has shown a significant increase in the number of scientific research in recent years, especially in the two years from 2019 to 2020, showing a strong momentum of development, and the number of published literatures in the world jumped from the sixth (2009-2019) to the second (2020), second only to the United States.
On February 23, 2021, the official website of the Food and Drug Inspection Center of the State Drug Administration issued the "Technical Guidelines for Non-clinical Research and Evaluation of Genetically Modified cell Therapy Products" (draft for comment), which mentioned the selection of animal species/models, when there is a lack of relevant animal models, Cell - and tissue-based models (such as 2D and 3D tissue models, organoids, and microfluidic models) that simulate the human environment in vivo can also provide useful complementary information for efficacy and safety assessments. With the deepening of organoid research and the continuous improvement of policies, organoids will play an increasingly important role in translational medicine and clinical individualized therapy.
With reference to the European organoid development model, it can be expected that the improvement of China's basic scientific research accumulation will accelerate the process of organoid industrialization, and we will also see the emergence of more organoid companies in the near future.
Table 1. Part of domestic and foreign organoid companies


Figure 11. Domestic track financing history

Figure 12. Financing history of foreign track
From the point of view of the number and amount of investment and financing, the entire organoid industry is still in a relatively early stage, and the organoid industry has not yet formed a centralized industrial cluster in China. The competition has just started, and enterprises with core technology advantages and complete production chains, as early as possible to lay out the industry will have a first-mover advantage.
Another development opportunity is that at present, domestic and foreign industries have not established perfect standards, so Chinese organoid companies and research institutions can actively participate in the standardization of organoid technology and the establishment of application guidelines, and can grasp the leading advantages and discourse rights in the industry in the future.
7 Latest scientific research progress
7.1 3D printing microfluidic chips
In a paper published in Lab on a Chip on April 12, 2022, Idris Salmon et al., from the Laboratory of Bioengineering and Mormorphology in the Department of Biodynamics, Department of Mechanical Engineering, KU Leuven, have developed a method based on human pluripotent stem cells to generate organoids that interact with blood vessel cells in a spatially determined manner. This 3D printable platform is designed to be compatible with any organoid system, opening new avenues for understanding and manipulating the co-development of tissue-specific organoids and vascularization [Lab Chip. 2022, 22(8):1615-1629].

Figure 14. 3D printed microfluidic platform for on-chip vascularized organoid culture

Figure 15. Vascular network characterization and organoid invasion in 3D printed microfluidic chips
7.2 Beta cell organoids in vitro
Published April 8 in the Nature Protocols literature, Jingqiang Wang et al. isolated islet progenitor cells from adult mice, enabling efficient generation and long-term expansion of functional islet organoids in vitro. His team achieved functional maturation of islet organoids by prolonging the culture period and circulating glucose stimulation. The obtained organoids are mainly composed of β cells, but also contain a small number of α, δ and pancreatic polypeptide cells. This method provides a strategy for generating beta cells in vitro and an organoid model for studying islet regeneration and related diseases [Nature Protocols. 2022, 17, 1359-1384].

Figure 16. Characterization of islet organoids in vitro and organoid cells in vivo
7.3 Brain autism model
On April 5, 2022, the Austrian Institute of Science and Technology discovered mutations in high-risk genes for autism and how they disrupt important developmental processes in the brain, using miniature brain models to help us understand autism. In contrast to previous models using mice, this study achieved great progress using brain organoids, concluding that the CHD8 mutation disrupts the balance of neuronal production, resulting in brain hypoplasia in patients [Cell Rep. 2022, 39(1):110615].
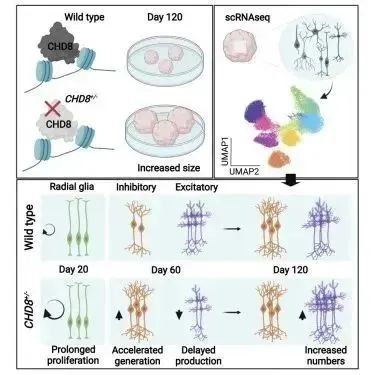
Figure 18. Mutant organoids overgrowth in control experiments
8. organoid technology challenges
The key technical bottleneck of organoids is the inability to achieve simultaneous growth of volume and function, and to solve this problem, we must first solve the main problems, including culture mode, vascularization and quantitative research.
1. Vascularization. At present, most organoids do not have the structure of vascularization. Therefore, as the size of the organoids grows, the organoids are limited by the lack of oxygen and the increase of metabolic waste, which may lead to tissue necrosis. Studies have been conducted to construct tumor organoids with vascular endothelial cell microenvironment, and co-culture organoid tumor cells and vascular endothelial cells on Matrigel to generate vascular structures in order to solve the problem of missing vascularization of organoids.
2. Immunization. Beyond vascularization, the challenge involves modeling the interaction between the tumor and the immune environment. In 2019, the Nature Protocol published related Protocols for co-culture of tumor organoids and immune cells, which can reflect and simulate part of the characteristics of tumor microenvironment [Nature Protocols. 2020, 15:15-39]. Taking the co-culture model of upper skin organoids and immune cells as an example, the interaction between organoids and immune cells can be reshaped by adding activated immune cells in the medium, co-growing with immune cells after tissue digestion into single cells, and adding recombinant cytokines in ECM.
3. Be systematic. Compared to individual organoids, the construction of organoid systems can provide a more complete and comprehensive assessment of drug efficacy and potential toxicity. At present, organoids can only detect the inhibitory effect of drugs on tumors, and can not predict the existence of other side effects and safety risks in other organs and tissues. In order to solve this problem, in 2017, Skardal et al. Constructed an organoid system composed of heart, lung and liver integrated in a closed circulation concern, so as to fully reveal the toxicity and efficacy of drugs on different organs [Sci Rep. 2017, 7(1):8837].
From the perspective of clinical application, organoids are difficult to perfectly simulate the full function of the original tumor. Tumor tissue is highly heterogeneous and complex in the human body, but for key indicators that predict drug susceptibility (such as cell inhibition rate), organoids only need to reach a certain degree of complexity to give a good answer.
In terms of vascularization, the organoids in culture to about 2 months, if the lack of nutritional supply, will form a large difference with the internal organs, but for the drug screen as long as the organoids grow to the cell ball in the right environment can be used for the drug screen.
For example, if the research focus of a drug is the need to cross the blood-brain barrier, then the construction of brain organoids will focus on the need to have a complete blood-brain barrier structure, and other characteristics (such as the interaction of cells and peripheral blood vessels) may not be prioritized.
The realization of vascularization, immunococulture and systematization can further improve the accuracy of clinical prediction of organoids, but considering the key application factors such as cycle and cost, all conditions cannot be taken into account. One day, if these features can be achieved with a manageable cost cycle, organoid drug screens will be able to provide more accurate answers.