
Source: medicine smelling window
Gene editing is expected to cure congenital diseases
With the completion of human genome sequencing and the emergence of efficient gene editing technology, quasi-disease treatment at the gene level has gradually come into the public's field of vision.
Gene editing mainly refers to the modification of nucleic acid sequence DNA or RNA by means of genetic engineering, so as to realize the directional changes of target genes, such as gene insertion, deletion and replacement, and finally obtain the required new traits. Gene editing therapy brings hope for solving the congenital diseases that have plagued human beings for a long time. Since many congenital diseases are caused by genetic abnormalities, human beings have long been unable to cure this type of disease. Gene editing can go deep into the source of the disease and bring hope of cure.
01
Gene editing brings hope to congenital diseases
Gene is an important factor in determining biological characterization. Genes are generally understood as carriers for storing biological genetic information. The existence of genes enables organisms to retain dominant representations, which is the basis for the emergence of advanced organisms. However, genetic inheritance is also accompanied by a large number of uncertainties, uncertainty comes from the randomness of biological activities, uncertainty brings the possibility of evolution, but also brings the risk of disease. For most sexually reproducing organisms, the genes are half from the father and the other from the mother, so offspring may cause diseases that have never been seen in their parents because of homozygous or heterozygous genes.
In human history, genetic diseases are often difficult to cure. Because the gene is hidden in the deepest part of the human body, it is difficult to solve the problem from the source, so it is usually necessary to take long-term medication to control the progress of the disease, and the disease burden is heavy. At the same time, many genetic diseases are rare, and the treatment options are very limited, resulting in higher drug prices and lower accessibility, which belong to unmet medical needs.
With the continuous progress of gene editing tools, gene editing therapy has also entered the fast lane of development. TALEN, ZFN, and the CRISPR-Cas system, which has revolutionized almost the entire field of gene editing, make gene editing therapy within reach. In 2020, Professor Jennifer Doudna of the University of California, Berkeley and Emmanuelle Charpentier of the Planck Institute won the Nobel Prize in chemistry for their outstanding contributions in the field of CRISPR. At the same time, CRISPR Therapeutics, founded by Professor J. Doudna as co-founder Intellia Therapeutics,E. Charpentier as co-founder, has grown into a leader in the field of gene editing therapy. Both companies have pipelines into the clinical stage, which is expected to bring dawn for the treatment of gene diseases that have plagued mankind for a long time in the near future. At the same time, based on gene editing technology, the ascendant cell therapy is expected to usher in a great-leap-forward development.
02
DNA defects are associated with a lifetime and may affect future generations
DNA affects the activity and characterization of the body through protein control. The process of transforming DNA into protein is divided into two steps: the first step is the conversion of DNA to mRNA, which is called transcription, which occurs in the nucleus; the second step is the conversion of mRNA into protein, which is called translation, which occurs in the cytoplasm. MRNA is the intermediate of DNA into protein, which is the origin of its name, namely messenger RNA (messenger RNA).
Generally speaking, DNA is similar to a manuscript, and changes in DNA will always exist in the body, and new cells that divide will inherit these changes, so there is a high probability that changes in DNA will be accompanied by a lifetime, in which changes in DNA in sex cells can even be passed on to the next generation. At the same time, some DNA fragments will affect the production rate and expression of mRNA. MRNA is similar to instructions and can guide its own cells to produce specific proteins. Protein is the tool obtained in the final production, which has a direct effect on the indicators of individual organisms. Changes in mRNA or protein are not inherited or inherited. This chain of transcription and translation is called the "central law" (The Central Dogma) of biology.
 Fig. 1 Central rule: DNA-mRNA- protein transformation process
Fig. 1 Central rule: DNA-mRNA- protein transformation process
03
DNA and genetic diseases and hereditary diseases
According to the definition of the National Cancer Institute, hereditary diseases (hereditary syndrome) usually refer to diseases caused partly or completely by hereditary genetic or chromosomal abnormalities. According to the definition of the National Institute of Human Genomics, genetic disease (genetic disorder) refers to a disease caused entirely or partly by abnormal DNA sequences. Although genetic diseases and genetic diseases are not exactly the same, they contain a lot of overlapping diseases.
As the storage carrier of human genetic material, DNA is hidden in the nucleus. On the one hand, it is protected by layers of cellular structure and human immune mechanism, so it is not easy to change; on the other hand, when there are genetic abnormalities, the existing medical technology is also difficult to provide effective treatment. At the same time, DNA abnormalities usually stay with the patient for a lifetime and are likely to be passed on to future generations. Therefore, patients have a heavy burden of disease and lack of effective treatment.
In addition, many rare diseases are caused by genetic defects. Most defective genes make it difficult for gene carriers to survive. Because genes are carriers of genetic information, it is difficult to inherit these genes in natural selection for a long time, so many genetic diseases / genetic diseases belong to unmet medical needs.
04
The working principle of gene editing
Gene editing tools give humans the ability to change DNA or RNA directionally. By inserting, deleting and replacing nucleotides at specific sites, we can change gene expression and then affect the protein sequence or structure for a long time. Citing the article "Genome Editing: Past, Present, and Future" published on Yale Journal of Biology and Medicine, human beings have been exploring ways to change genes throughout history. In the 20th century, human beings used chemotherapy and radiotherapy to change the DNA of tumor cells and disrupt the life activities of tumor cells, so as to eliminate tumors. However, the gene changes caused by these two methods are random, and it is difficult to locate accurately. With the development of science and technology, scientists have invented three important gene editing tools: ZFN, TALEN and CRISPR. The emergence of modern gene editing tools not only accelerates scientific research, but also brings possibility for the treatment of many genetic diseases.
Precise location is an important ability of gene editing and the first step to achieve directional editing. Targeting a specific target sequence, rather than changing it anywhere in the DNA, makes gene editing a controllable tool and avoids the risk of uncontrolled gene mutations. Uncontrolled genetic mutations or inaccurate genetic modifications often lead to unpredictable malignant diseases such as tumors and immune diseases.
On the basis of accurate location, a good gene editing tool should also be able to identify and locate any set sequence. In this way, gene editing can be widely used in different genetic diseases.
Many genetic diseases are caused by misexpression or overexpression of DNA. Through gene knockout or inhibition, a better therapeutic effect can be achieved. Therefore, only relying on gene editing tools for location, while the introduction of controllable enzymes at this site for cutting, can greatly alleviate or even cure this kind of disease. At present, most of the in vivo gene editing therapy makes use of this mechanism to knock out the pathogenic genes to achieve the purpose of treatment.
With the development of biotechnology, scientists have begun the research and development of gene knock-in. Compared with gene knockout, gene knockout is more complex, which often requires gene editing tools and gene insertion techniques (such as viral vector insertion), so the current progress lags behind gene knockout therapy. But gene knockout brings more possibilities to cure diseases, especially for some diseases caused by too little or lack of gene expression.
05
Gene editing has the potential to be permanently effective.
Although the names are similar, gene editing (gene editing) is essentially different from gene therapy (gene therapy).
Gene editing, by modifying the gene to correct the wrong gene. Because this change occurs in the original genome, even if the cell divides, the change will be inherited. Therefore, gene editing is generally considered to have the potential to permanently correct pathogenic genes. Furthermore, if the change occurs in sexual cells, it is likely to be inherited by future generations.
Gene therapy, on the other hand, delivers additional genes into cells so that cells can express additional genes without changing pre-existing genes. New genes will not be integrated into the original genome, so this change will not be inherited after the cell divides. In theory, gene therapy is slightly less durable than gene editing.
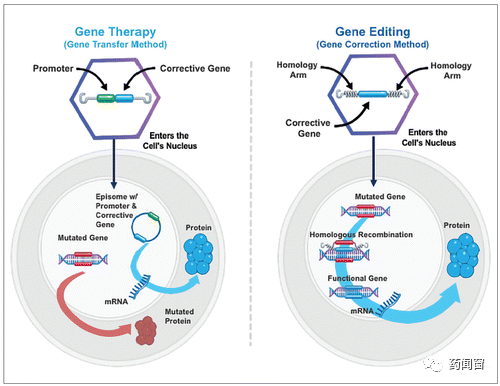 Fig. 2 Gene therapy vs Gene editing therapy
Fig. 2 Gene therapy vs Gene editing therapy
06
Gene editing tool: CRISPR
Human beings have been exploring biomedicine continuously, and the kinds of drugs they have mastered are constantly rich. From small molecular chemical drugs to biological drugs, nucleic acid interfering drugs (RNAi), gene therapy, gene editing therapy. With the abundance of drugs, there are more targets for treatment: small molecular chemicals and biological drugs (monoclonal antibody, double antibody, etc.) act on proteins, RNAi acts on RNA, gene therapy and gene editing act on DNA.
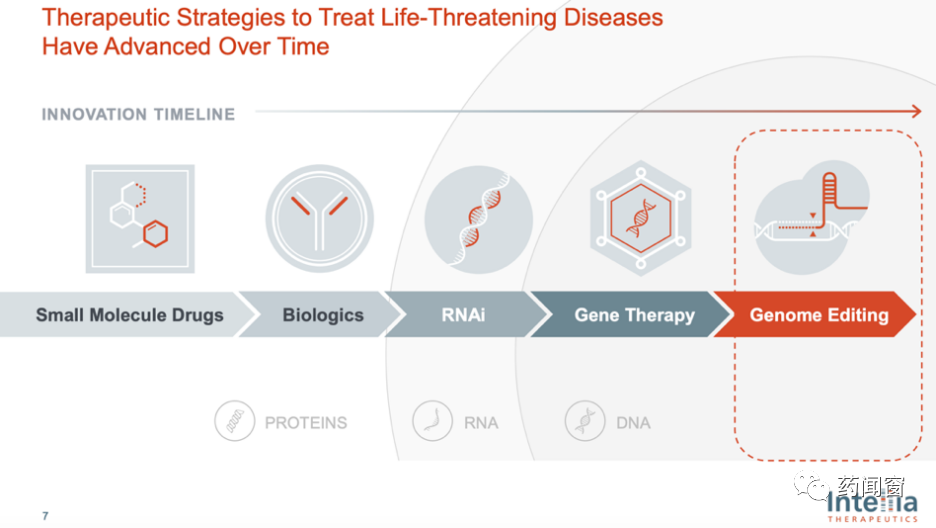 Fig. 3 Drug iteration history
Fig. 3 Drug iteration history
TALEN,Transcription Activation-Like Effector Nucleases is a kind of restriction enzyme. After engineering modification, it has the ability to cut specific sites of DNA chain. TALEN is mainly divided into two functional domains: DNA binding domain (DNA binding domain) and DNA cutting domain (DNA cleavage domain).
The DNA binding domain is responsible for identifying the site and guiding the cleavage enzyme to the correct editing site. The core part of the DNA binding domain consists of several linked repeated amino acid sequence fragments (tandem amino acid repeats), each of which consists of 33-34 units of amino acids, and each fragment corresponds to one base on the DNA chain. The sequence of each amino acid fragment group is basically the same, except for the difference between the 12th and 13th amino acids, these two sites are called repeat-variable diresidue (RVD). Because the amino acids of these two positions are variable, gene editing tools can target four different bases of DNA by controlling the amino acids of these two positions. Each fragment group corresponds to one DNA base, and several groups connected before and after can target specific DNA sequences.
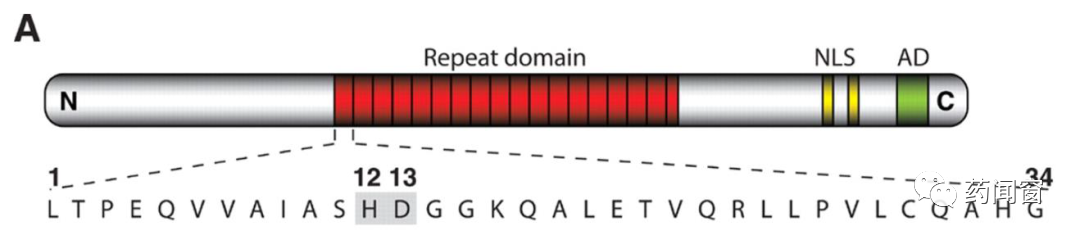 图4 TAL Effector结构
图4 TAL Effector结构
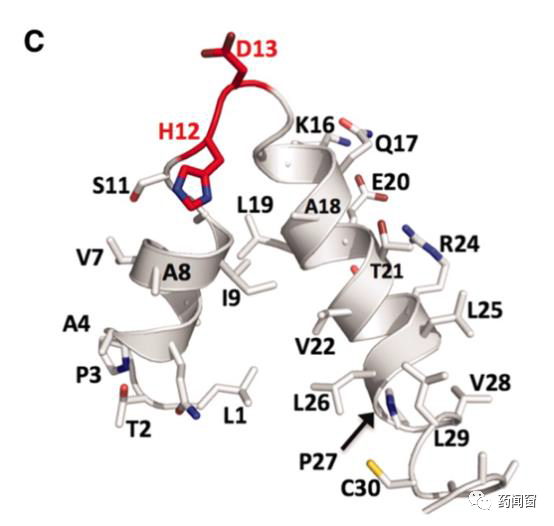 Fig. 5 schematic diagram of the three-dimensional structure of a repeat unit in TAL Effector
Fig. 5 schematic diagram of the three-dimensional structure of a repeat unit in TAL Effector
With the help of DNA binding domain, DNA cutting domain comes to the selected site for cutting. FokI endonuclease is a common cutting tool. FokI needs to form diploid (dimer) for cutting, so TALEN usually consists of two TALEN targeting the positive and anti-strands of DNA, respectively. TALEN will cut the position between the two targeting sequences.
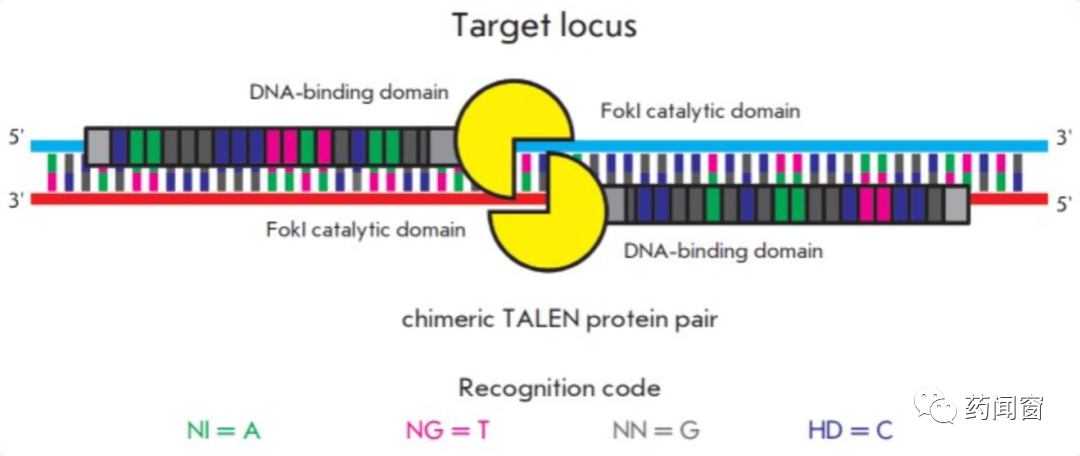
图6 TAL Effector + FokI 二倍体
Advantages:
Compared with ZFN,TALEN, it is more targeted and less toxic (probably due to more accurate affinity).
Compared with ZFN,TALEN without selection or directed evolution, it saves construction time and cost.
Compared with CRISPR,TALEN without PAM sequence, there are more targets that can be selected in theory.
(However, this advantage is not prominent because there are usually multiple potentially effective targets in the genome.)
Disadvantages:
TALEN has a large structure, which puts forward higher requirements for delivery.
Common viral vectors lentivirus (lentivirus) and adeno-associated virus (AAV) are difficult to transport "goods" with such a large molecular weight.
Scientists are currently trying to deliver TALEN in the form of mRNA.
Compared with CRISPR,TALEN, the manufacturing process is relatively complex and the cost is high.
Compared with the structural design of CRISPR,TALEN, it is more complex.
CRISPR only needs to reconstruct the gRNA sequence, while TALEN needs to consider factors such as DNA binding.
CRISPR,Clustered Regularly Interspaced Short Palindromic Repeat, derived from bacteria and other microorganisms.
CRISPR is an important immune mechanism for bacteria to resist virus invasion. by identifying virus gene sequences, CRISPR can guide the biological mechanism in bacteria to destroy and destroy virus gene sequences, so that viruses can not replicate and reproduce in bacteria.
CRISPR is a region in the bacterial genome composed of short DNA repeats (palindromes) and spacer. When a certain kind of virus invades for the first time, some genetic fragments of the virus are integrated and stored in spacer. After transcription and treatment of various proteins, CRISPR RNA (crRNA) with viral gene sequence is formed, and the molecular cutting mechanism is guided to cut the viral gene. Because the sequence is directly copied by the virus gene, the degree of recognition and match between crRNA and virus gene is very high.
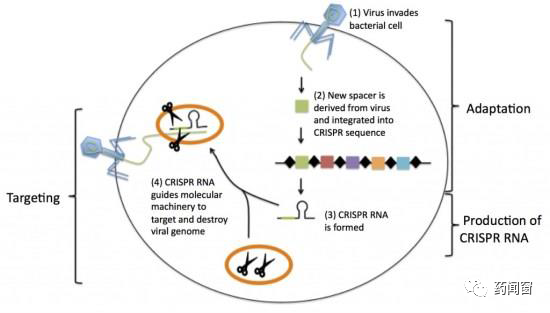 图7 CRISPR 对抗病毒入侵机制
图7 CRISPR 对抗病毒入侵机制
The CRISPR mechanism endows a reliable location mechanism. On this basis, a complete gene editing tool also needs a cutting mechanism to cut the DNA chain. In nature, the Cas family genes (CRISPR-associated gene) contained in the CRISPR immune system of bacteria can do this work. The Cas family contains many different genes with different functions or cutting methods.
GRNA is responsible for the locator of CRISPR gene editing. GRNA consists of crRNA and tracrRNA. The crRNA carries the RNA of the target sequence. CrRNA and tracrRNA form a complementary structure, and scientists further found that connecting crRNA and tracrRNA does not affect the operation and activity of the overall mechanism. The formed single-stranded gRNA (sgRNA) is more convenient.
Cas protein is the scissors responsible for cutting DNA strands. In Cas proteins, the HNH domain is responsible for cutting the target sequence, and the RVC domain is responsible for cutting the complementary chains of the target sequence. The cleavage site is related to the PAM sequence.
Previously, CRISPR could not work in mammalian cells because the CRISPR-Cas system could not cross the nuclear membrane and enter the nucleus. Zhang Feng's team added NLS (Nuclear localization sequence) to the Cas protein sequence, and NLS interacted with the nuclear vector to transport Cas into the nucleus.
Advantages:
Easy to operate, simple and convenient.
The design space is large and can basically target any target sequence.
The cost is low.
GRNA and Cas can split delivery.
Disadvantages:
The gRNA length of CRISPR is limited, which may lead to miss events.
07
Gene editing therapy pathway
According to the scene of gene editing, gene editing therapy can be divided into two pathways: in vivo (in vivo) and in vitro (ex vivo).
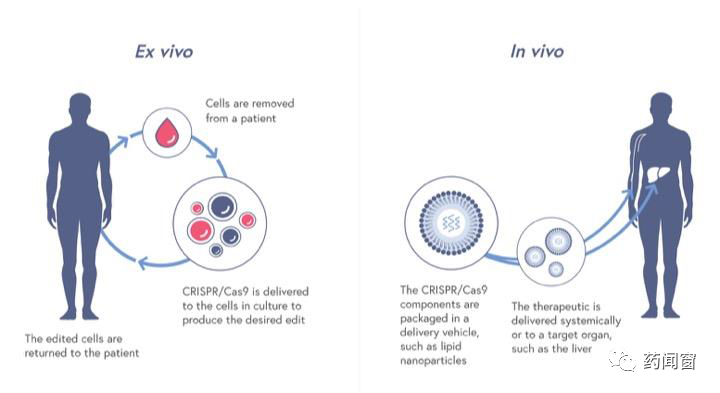 Fig. 8 two pathways of gene editing therapy
Fig. 8 two pathways of gene editing therapy
In vivo gene editing refers to the delivery of gene editing tools into the patient's body, and gene editing occurs in the human body. As the name implies, in in vitro gene editing therapy, gene editing takes place in vitro (such as the laboratory, etc.). It is usually necessary to extract patient cells, make gene editing of patient cells in the laboratory, and then transfuse the engineered cells back into the patient's body. The overall process is similar to existing CART cell therapy.
Each of the two paths has its own advantages and different technical challenges. At present, there are more companies using in vitro gene editing path.
This choice may be due to the higher safety requirements and technical difficulties of gene editing in vivo.In vivo gene editing is more difficult than in vitro gene editing, while in in vivo gene editing, gene knock-in is more difficult than gene knockout. Therefore, the technology required for gene knock-in is complex and demanding. Therefore, the current progress also lags behind other paths.
From a step point of view, gene knockout cuts the DNA strand at specific sites through the CRISPR system gRNA and Cas, resulting in double strand breaks (DSB), so that the target genes can not be expressed normally. According to the design idea of Intellia, gene knock-in also requires the CRISPR system to cut the DNA double strand first, and then insert the missing gene at the cutting position through the viral vector (Intellia currently uses adeno-associated virus AAV as the vector).
So the gene knock-in consists of two parts. In the first part, the LNP is used as the gRNA for positioning and the mRNA for encoding Cas (for cutting) in the delivery system, and the cutting is carried out at the set point. In the second part, the virus vector is used as the delivery system to deliver the patients' missing genes into the nucleus and integrate into the patients' genes.
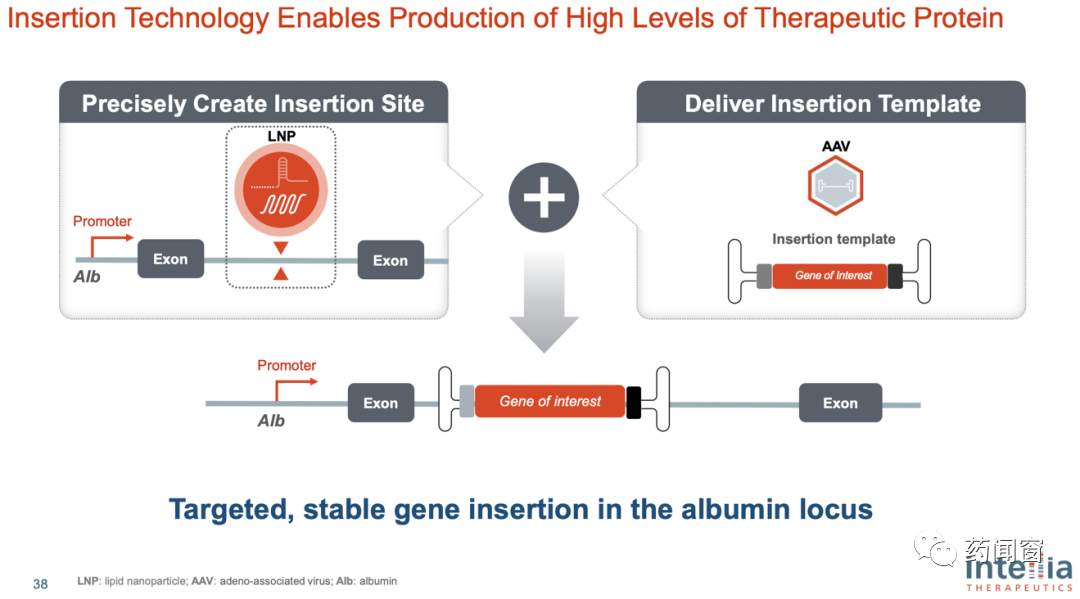
Fig. 9 realization path of gene editing-knock-in therapy in vivo
Compared with in vivo editing, in vitro gene editing is a subdivision track with more competitors. The main reason is that in vitro gene editing can ensure the quality of gene editing through quality control and remove substandard cells, which is more reliable from the point of view of safety and is more easily accepted by regulators.
From a certain point of view, in vitro gene editing is the use of gene editing tools to enable cell therapy. At present, cell therapy is subject to the mechanism of cellular immunity and is mainly autologous. Rejection comes from multiple human immune mechanisms, such as T cells and NK cells (associated with the HLA gene). At the same time, if the infusion of a large number of therapeutic cells, the infusion of cells may lead to rejection of human cells (related to TCR) (GvHD). Through gene editing, try to interfere or block the signal pathway that causes rejection.
Intellia provides some possible ideas for improvement here. For example, to avoid graft rejection host disease (GvHD) by knocking out the TCR of therapeutic cells, to enhance cell killing by introducing CAR or TCR by gene knock-in, to avoid CD-4-mediated rejection by knocking out II-type HLA gene, to avoid CD-8-mediated rejection by knockout HLA-A, and to adjust HLA-B and HLA-C to avoid NK cell-mediated rejection.