Source: The father of stem cells
Worldwide, the number of mesenchymal stem cell drugs that have been approved for marketing has reached 10. However, one of these products, South Korea's queencell, is controversial as to whether it is actually an MSCS drug. So, here we exclude it from the list.
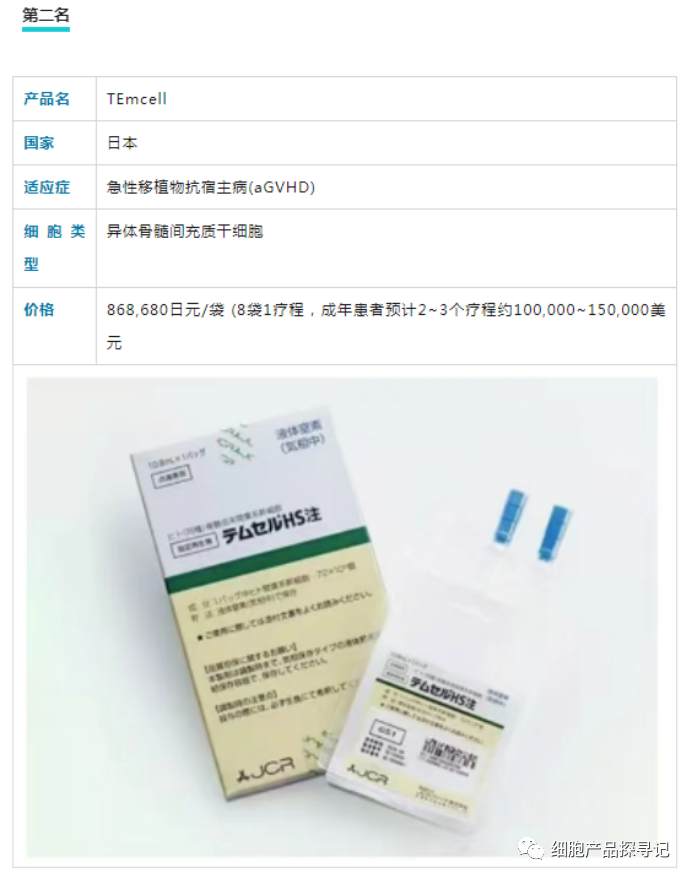
Of these MSCS that have received marketing approval, the most expensive is undoubtedly Prochymal, which is used to treat acute graft-versus-host disease. A course of the drug requires four doses, and its price tag is as high as $200,000.
In contrast, the lowest priced product is Stempeucel, a product that received a limited marketing license in India in 2016. Because its price is limited, its price is relatively low, only $2,200. By 2020, Stempeucel will be fully available in India. It may also indirectly reflect India's idiosyncratic approach to drugs. The prices are ranked from low to high as follows: