
Source:eMedClub
On September 20, 2023, a 58-year-old man named Lawrence Faucette received a genetically modified pig heart organ transplant, becoming the second patient in the world to receive a genetically modified pig heart. The FDA approved the experimental pig heart xenotransplantation under a "compassionate use" program. On Sept. 22, the University of Maryland School of Medicine (UMSOM) released a statement that Faucette was breathing on his own and that the new heart was functioning well without the help of any auxiliary equipment. Doctors at the University of Maryland School of Medicine believe that patients who receive xenotransplants are at greater risk, so they will closely monitor patients to detect any signs of ill health. How long the transplant of xenogeneic organs modified by gene editing can maintain the vitality of patients needs further observation and research.
Gene editing technology makes allotransplantation possible
There are two main types of organ transplantation, one is "allogeneic organ transplantation", such as organ transplantation between people; There is also "xenotransplantation", where organs are taken from animals and then transplanted into humans, which poses its own set of challenges but has the potential to save many lives. At the same time, xenotransplantation has the potential to spread disease from animals to humans, and there is also the risk that the recipient's immune system will reject the foreign body, which can be fatal to the patient. With the development of genetic technology, such as the emergence of gene editing technology CRISPR/Case9, it is possible to avoid a certain degree of immune rejection by knocking out and inserting a certain gene, making it possible to achieve a more matched xenotransplant donor.
Possible gene editing strategies involved in xenotransplantation of porcine hearts include: knocking out immunodominant heterologous antigen carbohydrate genes against immunogen (galactose-alpha-1, 3-galactose (GT), Sda blood group antigen (B4Gal), and n-acetylneuraminic acid (CMAH)); Knockout of the growth hormone receptor (GHR) gene to reduce the intrinsic growth of xenotransplantation; Complement regulation (CD46/ membrane coprotein (MCP)), anticoagulation (thrombomodulin (TBM) and endothelial cell protein C receptor (EPCR)), and anti-inflammatory (CD47 and heme oxygenase (HO-1)) were also expressed through transgenic expression.
World's first gene-edited pig heart transplant fails
In the world's first pig heart transplant for human surgery, scientists used gene editing to modify 10 genes, including knocking out one gene that controls organ growth and three genes that are rejected by human immune systems, while inserting six genes that make pig hearts better adapted to the human immune system.
On January 7, 2022, 57-year-old David Bennett received the world's first gene-edited pig heart transplant at the University of Maryland Medical Center (UMMC). On March 8, however, David Bennett died. The pig heart transplanted into David Bennett survived for only two months with no evidence of acute rejection.
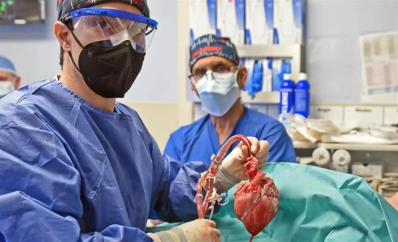
World's first gene-edited pig heart transplant
On June 30, 2023, the University of Maryland website reported that Muhammad Mohiuddin, professor of surgery at the University of Maryland School of Medicine and director of the Heart xenotransplantation program, and Bat Leigh Griffiths, a pig heart transplant surgeon, as the lead author, published a study in the medical journal The Lancet, which revealed the possible reasons for the failure of the world's first transgenic pig heart transplant: 1. The patient's poor health prior to transplantation limits the use of effective anti-rejection protocols used in preclinical studies of transplantation, while the patient's organs may be more susceptible to rejection by antibodies produced by the immune system. 2. In the second month after the transplant, the patient received two injections of intravenous immunoglobulin (IVIG) to help prevent infection and may also trigger an immune response to the pig heart. 3. There may be a latent virus in pig hearts called porcine cytomegalovirus (PCMV), which may be activated after patients reduce their antiviral treatment regimen, triggering an inflammatory response that leads to cell damage. However, there is no evidence that the virus infected patients or spread to organs other than the heart.
The world's second gene-edited pig heart has been transplanted into a human
As mentioned above, the focus of research remains in the direction of anti-immune rejection and antiviral infection, so in order to prevent the rapid rejection of pig organs by humans due to antibody-mediated rejection, the second transplant of pig heart organs receiving gene editing was performed using the donor pig to knock out three genes to eliminate alpha-gal sugars in pig blood cells. Avoid triggering immune rejection; Deleted a gene that prevents abnormal heart growth in pigs; The scientists also inserted six human genes to improve the acceptance of pig hearts by the human immune system. The donor pigs underwent 10 different gene edits. To avoid immune rejection, patients are also being treated with traditional anti-rejection drugs and a novel antibody therapy, tegoprubart, developed by Eledon Pharmaceuticals, in an animal model of xenograft rejection, tegoprubart has been shown to extend the time xenograft organs continue to function significantly.
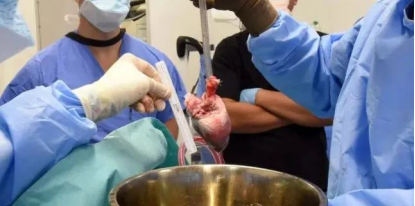
The world's second gene-edited pig heart transplant
For antiviral infection strategies, scientists at Revivicor, a biotech company that offers genetically engineered pig hearts, used a newly developed, more sensitive DNA test for the virus and tested tissue samples from donor pigs. The new PCMV antibody test starts from the two aspects of whether you are infected and whether you have been infected, to a greater extent to avoid still carrying latent pathogens deep in the cell.
Thoughts on gene editing/xenotransplantation
Gene editing relies on genetically engineered nucleases, also known as "molecular scissors," to generate site-specific double-strand breaks (DSBS) at specific locations in the genome, inducing the organism to repair the DSB via non-homologous end join (NHEJ) or homologous recombination (HR), as this repair process is prone to error, resulting in targeted mutations. This targeted mutation is called gene editing. The commonly used gene editing technology CRISPR/Cas9 system is mainly composed of Cas9 protein and single-stranded guide RNA (sgRNA), in which Cas9 protein plays the role of cutting double strands of DNA, sgRNA plays the role of guide, under the guidance of sgRNA through the principle of complementary base pairing. Cas9 protein can cut different target sites to achieve double strand breaks of DNA. The limitations of CRISPR/Cas9 technology are the low efficiency of homologous recombination, PAM sequence dependence, and off-target effects.
Will editing damage and possible off-target effects of gene editing technology affect the quality of modified xenografts and thus affect the success rate of allotransplantation? There are no definitive studies.
The reason why pig organs are selected for xenotransplantation is that the body temperature of pig and human is 36℃~37℃, the heart size, pipeline distribution and power output, and even the heart rate (55 ~ 60 times/min for pig and 60~100 times/min for human) are similar, and the risk of infectious diseases is low, and the number is relatively more abundant. In the medical world, pig heart valves implanted with human cells have been used in patient treatment, and pig ligaments and tendons have also been mature. The reason why there is no choice of primates with human "close relatives" is also a comprehensive consideration of many factors, most of the small primates, their organ performance and size can not bear the needs of human metabolism, while the larger body, itself in an endangered state, it is difficult to meet the number of human needs for organ transplantation. Primates have a low reproductive rate and can carry viruses that humans are susceptible to, such as simian immunodeficiency virus and Ebola virus, and once these organs are transplanted into humans, they can recombine to produce more harmful viruses.
X-ray machine diagram of human, monkey and pig
So far, two human transplants of gene-edited pig hearts have been carried out in humans, and we see that people have not paid much attention to the "life" of the pig heart itself, the average life span of pigs is about 15 years, so will the transplantation of pig organs accelerate human aging? Will the gene-edited pig heart further accelerate and shorten the "life" of the pig heart itself? Would it cause additional viral infections in humans if a gene-edited pig heart had to be transplanted multiple times? Will it accelerate the reduction of the patient's own immune resistance?
Peroration
While many questions remain about the future of gene-edited xenografts, as Dr. Muhammad Mohiuddin, a xenotransplant specialist on the University of Maryland team, put it: "It's amazing to see a pig heart working in a human body." "We don't want to predict anything, we're going to take every day as a victory and go forward."