
Source: Field of Cell and Gene Therapy
Over the past three decades, remarkable progress has been made in the field of gene therapy, from simple laboratory studies to approved drugs that offer hope to many patients with serious diseases. Dating back to more than 150 years ago, Mendel discovered genetic inheritance from the pea hybridization experiment, and back to today, gene therapy has been successfully applied and effective in clinical treatment.
Gene therapy products include all products that regulate the transcription or translation of target cells by transferring genetic material or that specifically alter the sequence of human genes, including nucleic acids (e.g., plasmids, ribonucleic acid transcribed in vitro), genetically modified viruses or microorganisms (e.g., viruses, bacteria, fungi), nucleases for specific editing of the human genome (e.g. CRISPR-Cas9, zinc finger nuclease) and genetically modified human cells in vitro.
Due to ethical and regulatory requirements, gene therapy products in clinical therapeutic research and clinical trials are limited to somatic cells. Thus, genetic material altered during gene therapy is not passed on to its offspring.
Gene therapy products used to treat human diseases are regulated as biologics. In 1974, the National Institutes of Health (NIH) established the Recombinant DNA Advisory Committee (RAC) to review and discuss gene therapy clinical trials, contributing to the risk regulation of gene manipulation techniques in the United States. In 2018, the NIH restructured the RAC's role to advise on issues related to emerging biotechnologies and renamed the RAC the Research Advisory Committee on Innovation and Special Technologies (NExTRAC) [1].
The world's first IND application for a gene therapy drug, submitted to the FDA in 1988, studied tumor-infiltrating lymphocytes (TILs) obtained from tumors of patients with advanced refractory melanoma after retrovirus-mediated gene modification was administered back to the patient in vitro. As a first step in the first gene therapy clinical trials, the first project only tested the likelihood of successful gene transfer and corresponding cell survival, rather than the therapeutic potential of gene correction. The clinical trial achieved its expected goal. In vitro gene transduction of autologous cells, in vitro expansion of genetically modified cells, and drug administration to patients under clinical conditions have all been shown to be feasible. After the modified gene injection, the cells survived, circulated in the blood, and homed to the target tumor site [2].
Nevertheless, the product later failed to prove its efficacy and the program was eventually terminated. However, the execution of these preliminary studies opens the way for subsequent gene therapy clinical trials. In subsequent years, new technologies for cell transfection and nucleic acid delivery continued to evolve, leading to more products entering the clinical stage (Figure 1). The number of gene therapy drug IND applications submitted to the FDA increased steadily between 1988 and 1999, followed by a marked decline and a nadir in 2002 (see Figure 1). The decline was due to a clinical side effect event in 1999 when one of the subjects, Jesse Gelsinger, died of liver failure shortly after receiving gene therapy. He is an 18-year-old participant in a clinical trial of a gene therapy drug based on an adenovirus vector that carries the normal ornithine transcarbamylase (OTC) gene for the treatment of X-linked OTC gene defects [3].
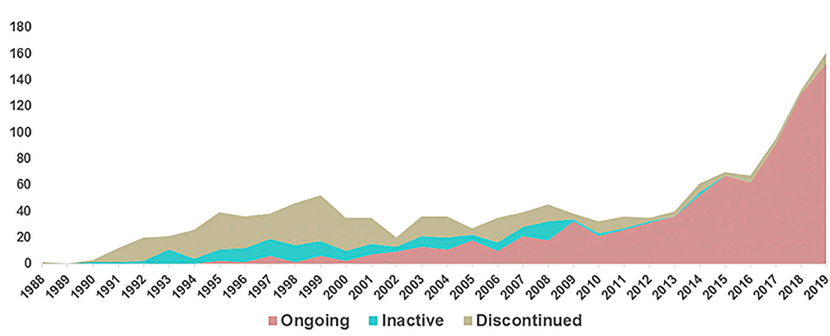 Figure 1. Number of gene therapy projects that submitted IND applications to the FDA from 1988 to 2019 [4]
Figure 1. Number of gene therapy projects that submitted IND applications to the FDA from 1988 to 2019 [4]
Of course, throughout previous history, the field of gene therapy has seen many failed products: some due to lack of efficacy, some due to severe side effects. In 1999, Jesse Kissinger's death from clinical side effects was one of the most tragic events in gene therapy clinical research, and this tragedy had a strong impact on the whole gene therapy field.
In response to this incident, the FDA and other stakeholders working in gene therapy have taken a number of steps to ensure that all gene therapy IND applicants strengthen the quality of their existing products and clinical trial oversight and monitoring. For example, in March 2000, the FDA issued a "Gene Therapy letter" to all applicants of a gene therapy IND, requiring them to submit their annual report summarizing all aspects of their product development, including product quality, manufacturing, animal testing, and clinical trials. Since then, the FDA has issued a series of guidance documents related to gene therapy products.
It took more than a decade for the gene therapy field to recover from this, during which time the number and growth of clinical trials worldwide was low. Although in 2003, the adenovirus vector gene therapy drug Gendicine was approved for marketing in China, China became the first country to approve the marketing of gene therapy drugs. However, due to the safety of gene therapy drugs, regulators are more cautious about gene clinical trials.
In 2012, the AAV gene therapy drug Glybera developed by UniQure was approved by the European Medicines Agency for the treatment of lipoprotein lipase deficiency, marking the dream of gene therapy "into reality." In subsequent years, clinical trial launches increased significantly, with record increases every year from 2013 to 2018 。
The largest increase in gene therapy clinical trial initiation occurred in the 2017-2018 period, as in August 2017, the FDA approved the first cellular gene therapy drug in the United States, Novartis' Kymriah, a lentiviral vector based in vitro gene therapy drug. It is the world's first approved CAR T gene therapy, marking the formal application of cell gene therapy technology represented by CAR T to cancer treatment, and in December of the same year, the FDA approved the first in vivo gene therapy drug Luxturna in the United States.
This has clearly boosted confidence in gene therapy. In 2019, FDA approved Zolgensma, an in vivo gene therapy drug used to treat SMA. Zolgensma's sales volume in 2021 was $1.352 billion [6], and the total revenue of Zolgensma in the first three quarters of 2022 was $1.37 billion [7]. This marks a huge commercial success for gene therapy.
Since 2022, in just over a year, more than a dozen gene therapy drugs have been approved for market, and the FDA has said on several occasions that they expect to approve 10 to 20 cell gene therapies per year by 2025.
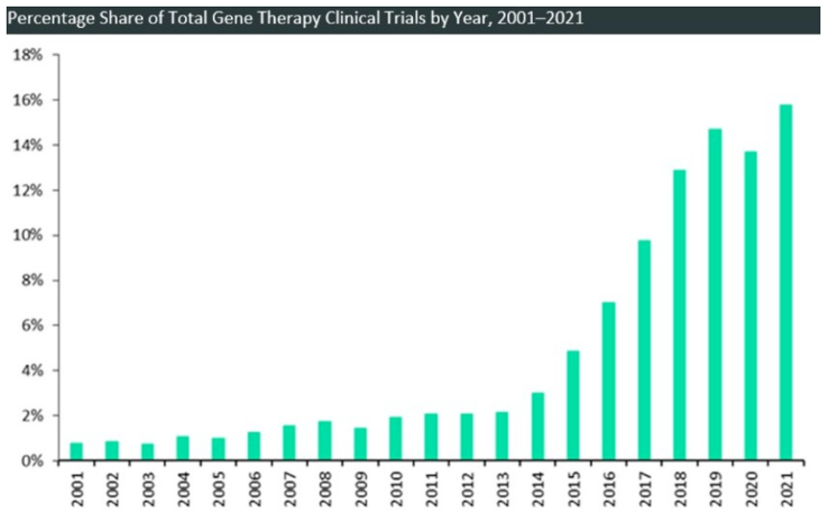
Compared with non-viral vector, due to the relatively efficient gene delivery efficiency of viral vector, the most commonly used vector type of gene therapy is viral vector. There are hundreds of viral vector-based gene therapy clinical trial projects around the world, of which about half use AAV vector. AAV gene therapy accounts for more than 40%. Research reports show that AAV gene therapy clinical trials have been growing rapidly in the past few years, from 1998 to 2007, there were only 22 publicly conducted AAV gene therapy clinical trials, and in the four and a half years from 2018 to June 2022, this number has doubled several times. The number of clinical trials reached 120 (see chart below). At least 22 of these trials have entered phase 3 clinical trials [8]. In addition, up to now, more than 20 domestic AAV gene therapy IND have been approved, of which 3 are in the clinical phase 3 stage [9]. These indicate that more and more AAV gene therapy drugs will be available in the next few years.
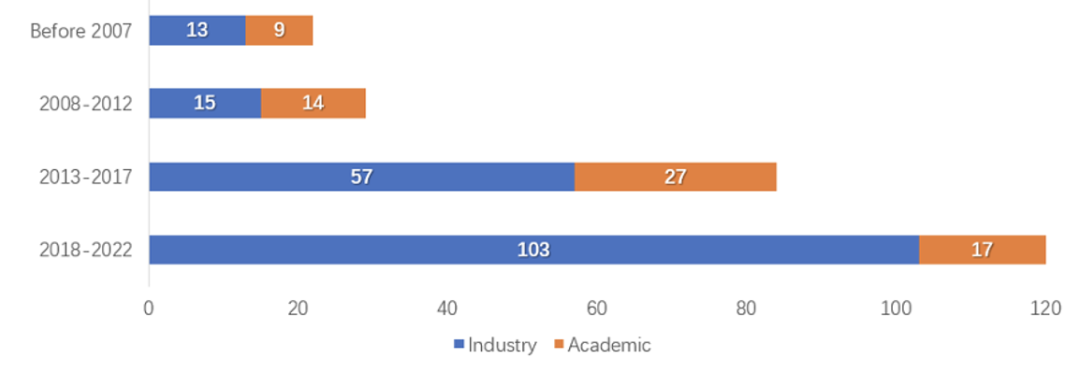 Figure 3. Number of AAV gene therapy clinical trial registrations in different periods [8]
Figure 3. Number of AAV gene therapy clinical trial registrations in different periods [8]
Although there is still some way to go before gene therapy becomes a routine treatment, more and more gene therapy has been approved for market, the number of clinical trials has increased sharply and the support of numerous documents and regulations indicates that gene therapy has stepped out of the "dark period" and is about to enter a period of rapid development.
Reference sources:
[1]https://osp.od.nih.gov/events/novel-and-exceptional-technology-and-research-advisory-committee-2/
[2]Cournoyer, D , and C. T. Caskey . "Gene transfer into humans: a first step." New England Journal of Medicine 323.9(1990):601-3.
[3] Wilson, James M. "Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency." Molecular Genetics & Metabolism 96.4(2009):151-157.
[4]https://www.cell.com/molecular-therapy-family/methods/fulltext/S2329-0501(20)30208-4?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2329050120302084%3Fshowall%3Dtrue
[5]https://www.globaldata.com
[6]https://www.novartis.com/news/media-releases/novartis-delivers-mid-single-digit-sales-growth-margin-expansion-and-advancement-robust-pipeline-2021
[7] https://www.novartis.com/investors/financial-data/product-sales
[8] https://www.frontiersin.org/articles/10.3389/fimmu.2022.1001263/full
[9] https://mp.weixin.qq.com/s/HEbbTHIKxwb8BGl0u7_MEw