Source: National Local Laboratory of Adult Stem Cells, Drug Examination Center of State Food and Drug Administration, Shanghai Huaxing Health Industry Cooperation Promotion Center
As of September 30, 2023, 88 clinical trial applications for stem cell drugs from 52 domestic enterprises (excluding subsidiaries) were accepted, of which 3 were added in August and 4 were added in September. A total of 69 of 44 companies (excluding subsidiaries) were granted tacit access to clinical trials (implied permission for clinical trials), of which 3 were added in August and 1 was added in September. Of the 88 accepted stem cell drugs, 10 have been unable to find IND review information or review suspended, and 10 are under review.
Added in August
New acceptance
1、Zhejiang Jinshidai Biotechnology Co., Ltd.
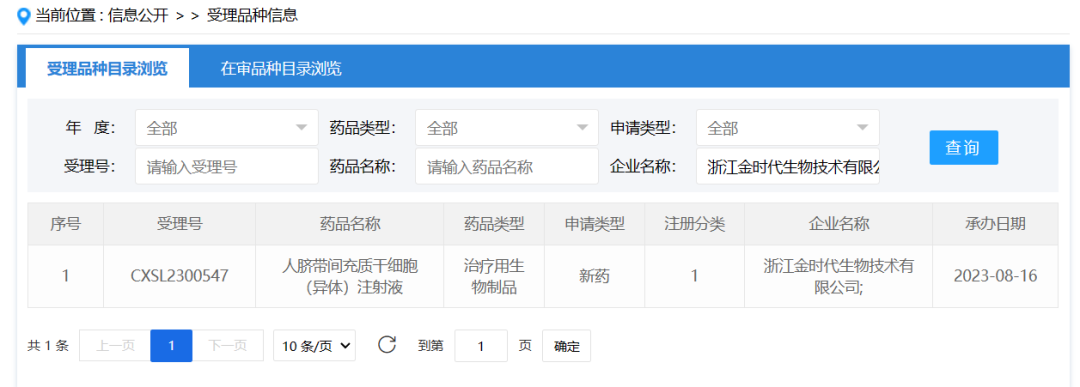
Zhejiang Jinshidai Biotechnology Co., Ltd., August 16, 2023. The clinical trial application for Human umbilical cord mesenchymal stem Cell (Allogeneic) injection was accepted (Acceptance number: CXSL2300547) for the treatment of acute graft-versus-host disease (GVHD).
Umbilical cord mesenchymal stem cells have strong immunomodulatory effect and promote hematopoietic recovery, which is expected to be used in the treatment of GVHD. In the pre-clinical safety and effectiveness evaluation work completed in the early stage, the research team found that human umbilical cord mesenchymal stem cell (allogeneic) injection (clinical proposed dose) had no toxic reaction after single or multiple administration to experimental animals, and had no side effects on the central nervous system, respiratory system and cardiovascular system. It is non-tumorigenic and has a significant effect on improving the symptoms of GVHD animal models.
2、Chengnuo Regenerative Medical Technology (Beijing) Co., Ltd.
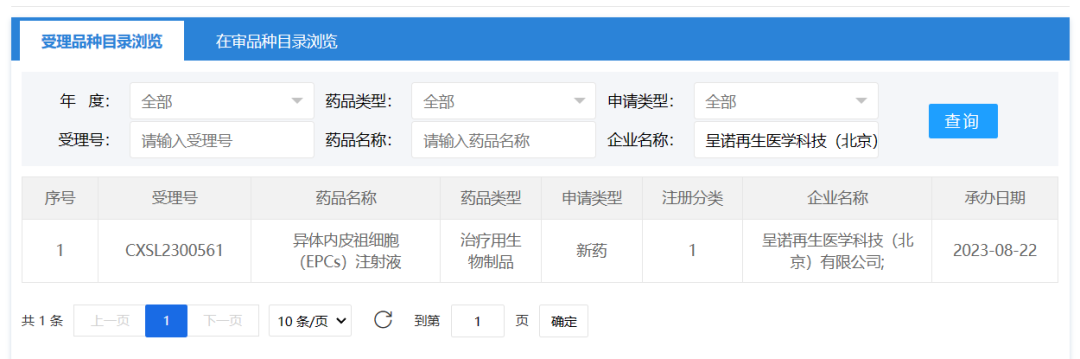
August 22, 2023, Chengnuo Regenerative Medical Technology (Beijing) Co., Ltd. The clinical trial application for allogeneic endothelial Progenitor cells (EPCs) injection (ALF201) was accepted (CXSL2300561).
ALF201 is an injection of allogeneic endothelial progenitor cells (EPCs) derived from induced pluripotent stem cells (IPscs). In April 2022, ALF201 injection (acceptance number: CXSL2200090) developed by Cino Medical received the implicit approval of CDE clinical trials, becoming the world's first approved clinical IPSC-derived cell candidate product for the treatment of acute ischemic stroke, and the IND application is a new indication for clinical application.
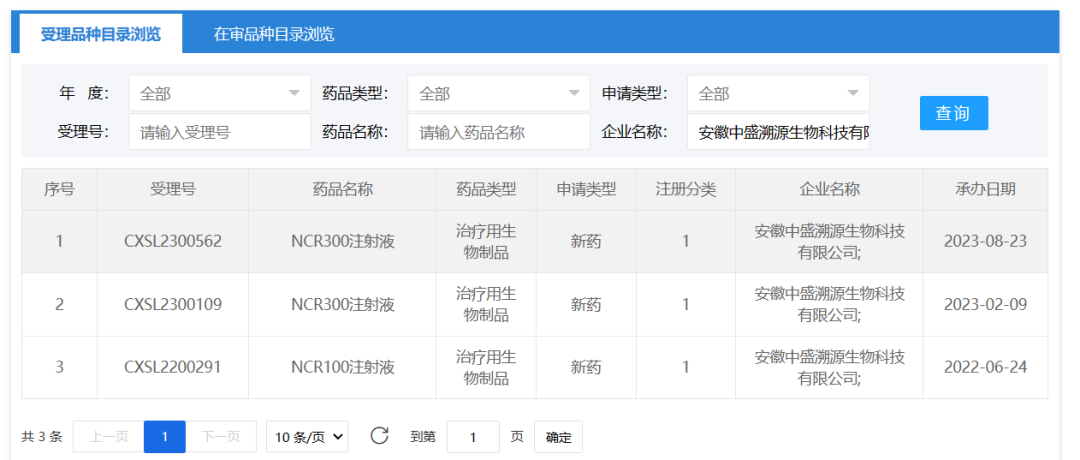
3、Anhui Zhongsheng Traceability Biotechnology Co., Ltd.
August 23, 2023, Anhui Zhongsheng Traceability Biotechnology Co., Ltd. The "NCR300 Injection" clinical trial application was accepted (CXSL2300562).
"NCR300 Injection" is the IPSC-derived natural killer cell (iNK) product prepared by industrial and large-scale production under the cGMP standard through optimizing the production process, which can be used in the clinical treatment of various malignant tumors and improve the efficiency and stability of cell therapy. On April 20 this year, the "NCR300 injection" sourced by Zhongsheng was approved for clinical use, and it is intended to carry out clinical trials for myelodysplastic syndrome (MDS), becoming the first approved clinical IPSC-derived iNK therapy in China. This clinical application is a new indication application.
Added implied license
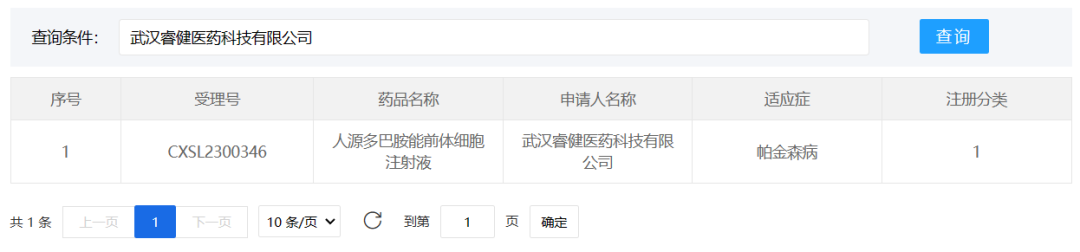
1、Wuhan Ruijian Pharmaceutical Technology Co., Ltd.
August 2, 2023, Wuhan Ruijian Pharmaceutical Technology Co., Ltd. NouvNeu001 (CXSL2300346), a new drug for Parkinson's disease, received implicit approval for a new drug clinical trial. This is also the first universal cell therapy product based on chemical induction to enter the clinical stage in the field of Parkinson's disease treatment in the world.
NouvNeu001 is a general-purpose cell therapy product that focuses on clinical needs and uses compounds to achieve fine cell function transformation, so as to truly meet clinical needs. NouvNeu001 realizes the differentiation of high-purity neuron subtypes through compound regulation, forms connections with the original neurons in the body, and enhances the cell secretion function, and further strengthens the improvement of the original lesions by transplanted cells, so as to achieve comprehensive therapeutic effects.
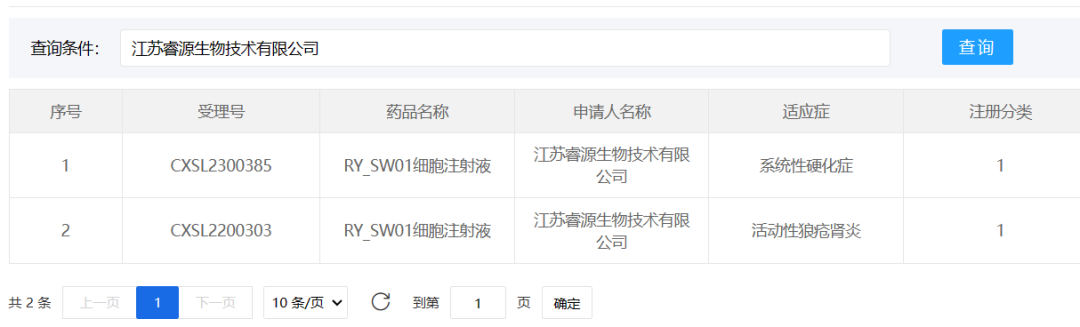
2、Jiangsu Ruiyuan Biotechnology Co., Ltd.
August 22, 2023, Jiangsu Ruiyuan Biotechnology Co., Ltd. "RY_SW01 Cell Injection", a new Class 1 biologics drug, was granted an implied approval for new drug clinical trials (CXSL2300385) for "systemic sclerosis". This indication is a rare disease and there is no effective treatment in clinic.
"RY_SW01 cell injection" belongs to mesenchymal stem cell therapy products. Compared with traditional therapy, mesenchymal stem cells can regulate immune through various pathways and secrete cytokines to achieve anti-fibrosis effect. In terms of safety, it will not cause adverse reactions similar to immunosuppressants. These properties of mesenchymal stem cells provide a new strategy for the treatment of scleroderma patients, which can reshape the patient's immune system to relieve pain and treat the disease.
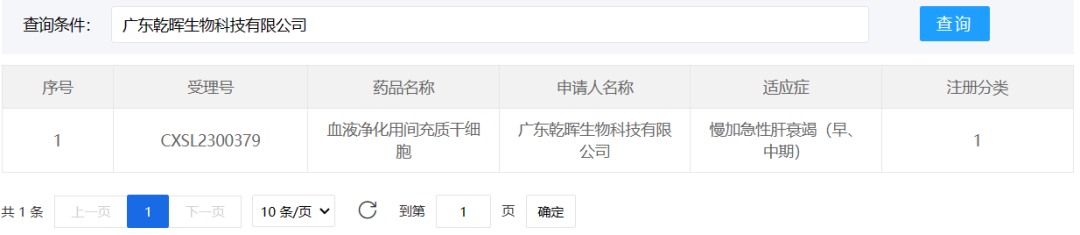
3、Guangdong Qianhui Biotechnology Co., Ltd.
August 29, 2023, Guangdong Qianhui Biotechnology Co., Ltd. "Mesenchymal Stem cells for Blood Purification" (Acceptance number: CXSL230079) received an implied approval for clinical trials of new drugs. This is the world's first blood purification mesenchymal stem cell product suitable for combined bioartificial liver therapy, the indication is: slow plus acute liver failure (early and middle stage).
Added in September
New acceptance
1、Ruijian Pharmaceutical Technology (Suzhou) Co., Ltd.
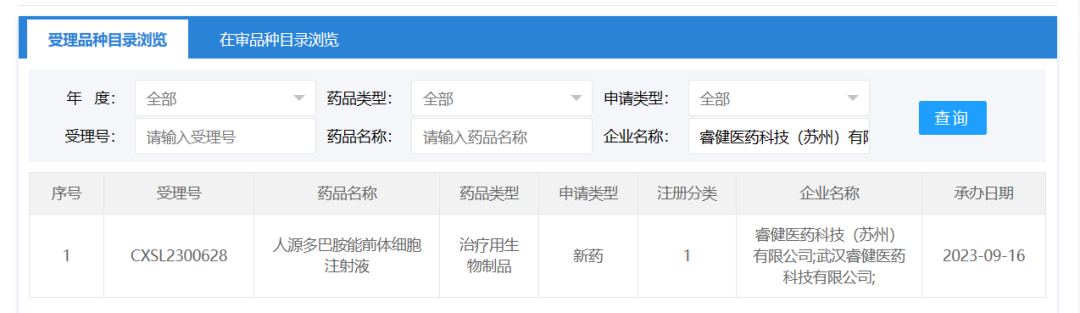
September 16, 2023, Ruijian Pharmaceutical Technology (Suzhou) Co., Ltd. A drug clinical trial application for Human Dopaminergic Precursor Cell Injection (IPSC-derived drug) was accepted (acceptance number: CXSL2300628). This is the second indication accepted for this cell injection, which has previously been implicitly licensed for Parkinson's disease (Acceptance number: CXSL2300346).
NouvNeu001 is an IPSC-derived drug for Parkinson's disease developed by Ruijian Pharmaceutical through its self-developed high-efficiency chemical small molecule induced functional cell regeneration technology. It uses induced pluripotent stem cells as "seeds" to grow healthy dopamine neural precursor cells, and then implants them into the lesion site to make up for the shortage of dopaminergic neurons. In addition to secreting dopamine, such precursor cells can further secrete a variety of proteins and small nucleic acids to improve PD lesions and provide a good "soil" environment for cells transplanted to the brain to play a role.
2、Beijing Yuge Stem Cell Technology Co., Ltd.
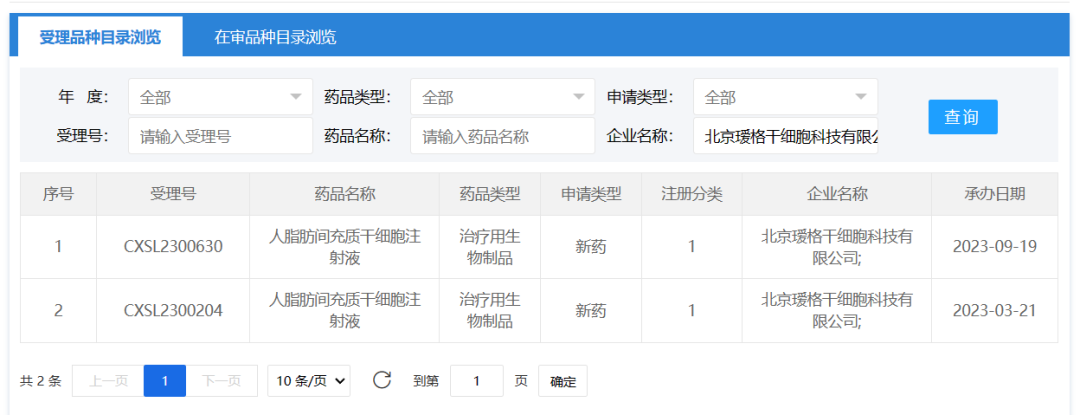
September 19, 2023, Beijing Yuge Stem Cell Technology Co., Ltd. A drug clinical trial application for "Human adipose mesenchymal stem cell Injection" was accepted (acceptance number: CXSL2300630). This is the second indication to be admissible for this stem cell injection, which has previously received implied approval for hand dysfunction (contracture) due to skin fibrosis of the hand in systemic sclerosis (Admissibility number: CXSL2300204).
AG1001 is a stem cell therapy drug that is introduced and developed based on the clinical research and patented technology of Peking Union Medical College Hospital. Strict quality control system and preparation technology are adopted in the development process to ensure the uniformity and stability of product quality and biological efficacy. Good safety and effectiveness data have been obtained in the preclinical study. The functional research and clinical trial exploration of AG1001 in immune regulation, anti-fibrosis and promoting angiogenesis, etc., can combat the three pathological axes of systemic sclerosis, and is a new drug with great potential for the treatment of systemic sclerosis, and has great clinical application potential.
3、ELP Regenerative Medicine Technology (Shenzhen) Co., Ltd.
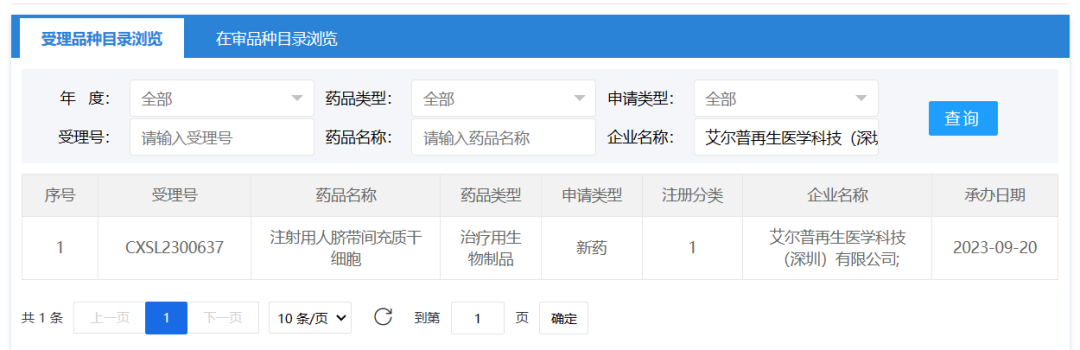
September 20, 2023, ELP Regenerative Medicine Technology (Shenzhen) Co., Ltd. A clinical trial application for "Injection of human umbilical cord mesenchymal stem cells" was accepted (accepted: CXSL2300637), which is the first mesenchymal stem cell drug of Elp. Epp has previously received tacit approval for human IPSC-derived cardiomyocyte injection (Acceptance number: CXSL2200547) for the treatment of severe chronic ischemic heart failure.
4、Shenzhen Yinguan Biological Technology Co., Ltd.
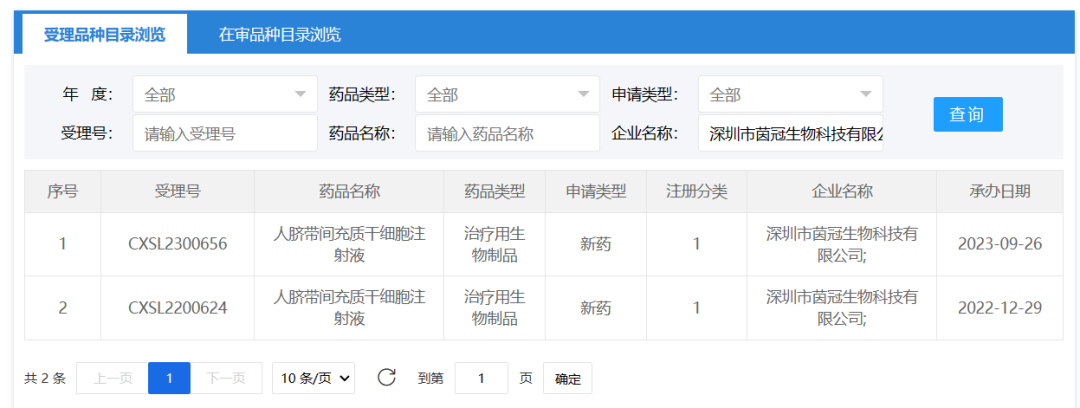
September 26, 2023, Shenzhen Yinguan Biological Technology Co., Ltd. A drug clinical trial application of "Human umbilical cord mesenchymal stem cell injection" was accepted (accepted: CXSL2300656), which is the second indication of this stem cell injection was accepted. Information about the drug has not been disclosed. It has previously been implicitly licensed for acute ischemic stroke (Acceptance number: CXSL2200624).
Added implied license
Wuhan Hamilton Biotechnology Co., Ltd.
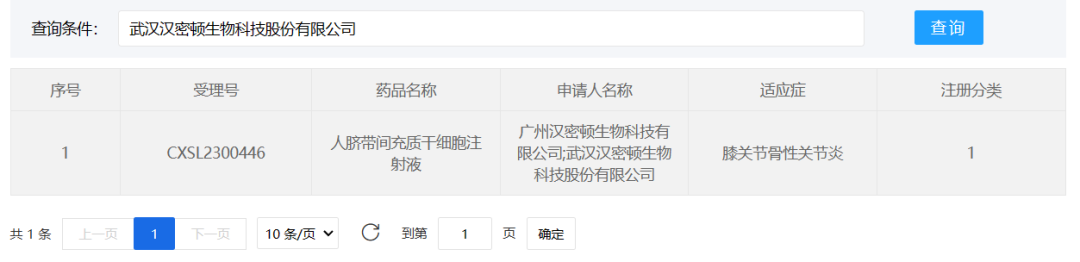
Wuhan Hamilton Biotechnology Co., Ltd, September 27, 2023. "Human umbilical cord mesenchymal stem cell Injection" (acceptance number: CXSL2300446) was granted implied approval for clinical trials of new drugs, and the indication was knee osteoarthritis. This is the first indication of the stem cell injection.