
Source: Cell and gene therapy field
At present, a total of 26 AAV gene therapy drug IND applications were approved, of which 3 entered phase III clinical. According to the IND approval time order is summarized as follows:
1. SKG0106 of Skyline Therapeutics
Skyline Therapeutics' AAV ophthalmic gene therapy SKG0106 was recently approved by the National Food and Drug Administration for neovascular age-related macular degeneration (nAMD). SKG0106 was approved by the FDA in June to begin a global Phase I/IIa clinical trial for the treatment of nAMD. SKG0106 is an innovative ophthalmic gene therapy drug under research. The therapy uses AAV as a carrier to overexpress anti-vascular endothelial growth factor (VEGF) protein and is administered by intravitreal injection, which is theoretically effective for a long time after last administration.
2. JNJ-81201887 by Janssen
In early August 2023, JNJ-81201887 (AAVCAGsCD59), an AAV gene therapy declared by Johnson & Johnson's Janssen, received implicit approval from the CDE clinical trial to be developed for the treatment of adult patients with geoglyphic atrophy secondary to age-related macular degeneration.
JNJ-81201887 (JNJ-1887, AAVCAGsCD59) is an AAV vector-based gene therapy in progress designed to increase expression of soluble CD59 (sCD59) and protect retinal cells, thereby slowing and preventing disease progression.
3. BBM-H803 of Belief BioMed
On July 24, 2023, Shanghai Belief-Delivery BioMed Co., Ltd, a wholly owned subsidiary of Belief BioMed. And Shanghai Mianyi Biotechnology Co., Ltd. The application for clinical trial of "BBM-H803 injection" was granted implied clinical trial approval for hemophilia A.
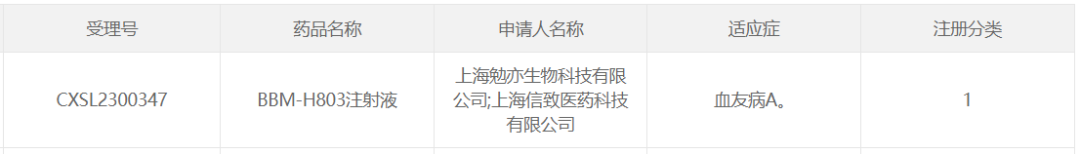
BBM-H803 is an AAV gene therapy drug owned by Belief BioMed with independent intellectual property rights, which introduces human coagulation factor Ⅷ gene into hemophilia A patients through intravenous administration, thereby improving and maintaining the level of coagulation factor in patients.
4. FT-004 of Fangtuo Biotechnology
July 12, 2023, Fangtuo Biotechnology (Suzhou) Co., Ltd. FT-004, an AAV gene therapy drug developed for hemophilia B (endogenous FIX activity ≤2%), was approved for CDE clinical trials.
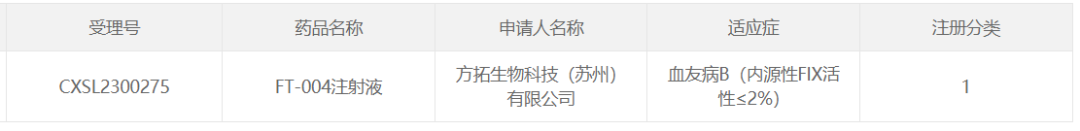
FT-004 is a gene therapy drug based on AAV carrier. Pre-clinical data show that FT-004 can efficiently enter liver cells, sustainably and stably express and secrete functional hFIX protein into the blood, which can effectively improve the clotting ability of model animals for a long time with good safety.
5. ZS802 of Sichuan Real&Best Biotech
June 28, 2023, Sichuan Real&Best Biotech Co., Ltd. The AAV gene therapy drug "ZS802 injection" for hemophilia A has been approved by CDE and is about to start clinical phase I/II trials.
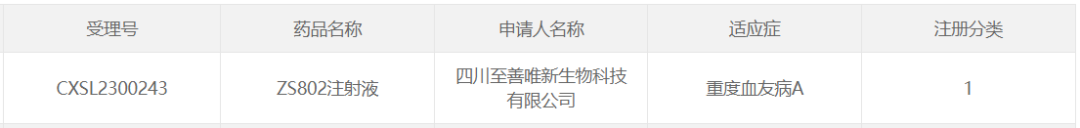
ZS802 is a company of Sichuan Real&Best Biotech Co., Ltd. The self-developed AAV gene therapy drug uses the world's smallest liver-specific promoter independently developed by the company, which solves the problem of large capacity of packaged genes and significantly improves product quality. In addition, ZS802 uses the optimized and modified FVIII gene sequence of ZS802, which effectively improves its efficacy. At present, ZS802 project has been exhibited in the Chinese Academy of Medical Sciences Hematology Hospital IIT study, the research results preliminarily proved the safety and effectiveness of the drug.
6. EXG102-031 of Hangzhou Jiayin BioTech
On June 1, 2023, Hangzhou Jiayin BioTech Ltd's AAV gene therapy drug EXG102-031 eye injection was approved by CDE for wet age-related macular degeneration (wAMD).
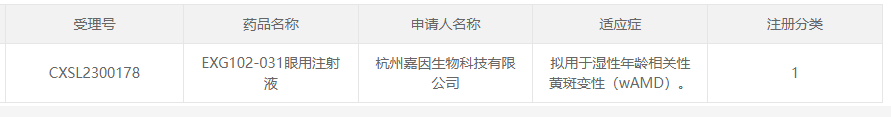
The therapy uses AAV as a carrier to overexpress a therapeutic fusion protein that binds/neutralizes all known vascular endothelial growth factor (VEGF) and ANG2 subtypes and is theoretically effective over a long period of time with the last dose.
7. AL-001 of Beijing Anlong Biomedical
April 27, 2023, Beijing Anlong Biomedical Co., Ltd. The company announced that its ophthalmic gene therapy product "AL-001 Ophthalmic Injection" IND has been approved by CDE for wet age-related macular degeneration (wAMD). On May 29, 2023, Anlong Biology announced that the project was approved by the institutional project establishment and ethics committee of Peking Union Medical College Hospital, the research leader, marking that the project officially entered the clinical trial stage.
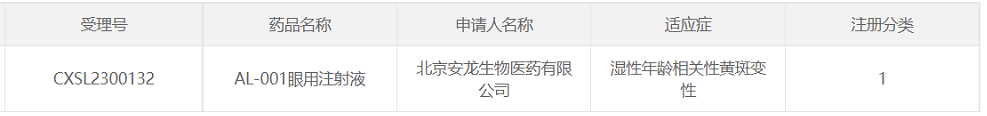
This therapy is the first gene therapy approved for wAMD in China by injection into the suprachorioidal space (SCS). The therapy uses AAV as the carrier to overexpress anti-VEGF protein, which is theoretically effective in the last administration for a long time. The product uses an advanced, self-developed rAAV production process for rhabavirus-free Sf9 suspension cells.
8. HG004 of Huigene Therapeutics
April 18, 2023, Huigene Therapeutics Co., Ltd. The company announced that the IND for HG004, the first ophthalmic AAV gene therapy drug developed by the company, has been approved by the CDE for the indication: Leber's congenital amaurosis type 2 (LCA2), and in January this year, HG004 has received IND approval from the FDA.
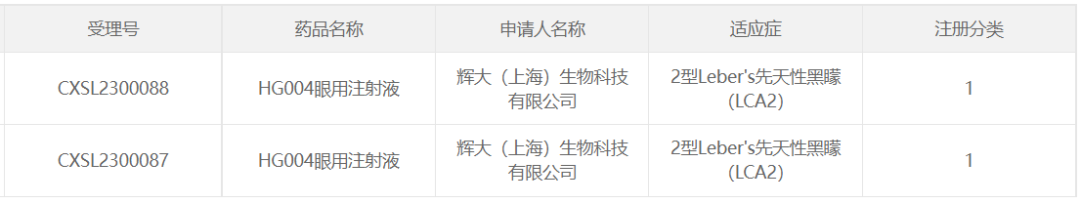
HG004 is an AAV-mediated gene replacement therapy designed to treat retinopathy associated with mutations in the RPE65 gene, which can lead to severe early-onset childhood retinal dystrophy, early-onset severe retinal dystrophy, Leber congenital amaurosis (LCA), or retinitis pigmentosa (RP), among others. The AAV vector used in HG004 has a transduction rate against retinal pigment epithelium at least 10 times higher than that of AAV2, and the initial effective dose is much lower than that of the marketed AAV2-HRPE65 (LUXTURNA). In the IIT clinical study conducted by Shanghai Xinhua Hospital in China, HG004 has achieved good clinical efficacy, and the patient's vision has been significantly and substantively restored.
9. NFS-02 of Wuhan Neurophth Biotechnology
On April 14, 2023, Wuhan Neurophth Biotechnology Limited Company's AAV gene therapy drug NFS-02 eye injection IND applied for the implied license of CDE, with the indication of Leber hereditary optic neuropathy (G3460A). In addition, NFS-02 received a New Drug Clinical Trial (IND) approval from the FDA last year.
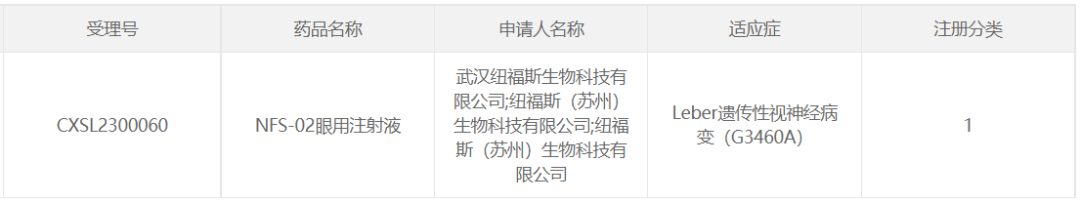
NFS-02 is a new intraocular injectable gene therapy drug based on AAV2. The therapeutic gene can be delivered to the damaged optic ganglion cells of the patient through a single intravitreal injection of the drug, repairing the biological respiratory chain of the mitochondria damaged by gene mutation, and then restoring the visual function of the optic ganglion cells.
10. GS1191-0445 of Suzhou Huayilajian Biotechnology
On January 16, 2023, Suzhou Huayilajian Biotechnology Co., Ltd. GS1191-0445 injection, an AAV gene therapy drug, was approved by CDE for clinical trials for the treatment of hemophilia A.
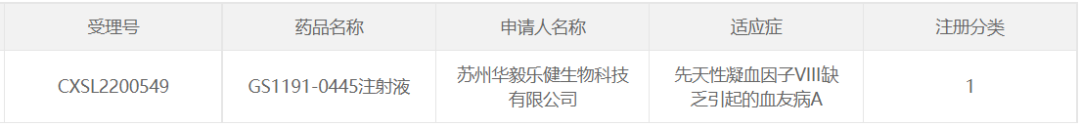
Suzhou Huayilajian Biotechnology Co., Ltd. In November 2022, the IND application for the therapy was officially submitted to the CDE and was accepted, becoming the domestic hemophilia A treatment drug entering the IND stage. In addition, the IIT study of this drug was carried out in Tianjin Blood Research Institute, and 9 patients were enrolled, and good safety and exciting efficacy data were obtained after administration.
11. LX102 of Longxin Qisheng (Suzhou) Biopharmaceutical
December 23, 2022, Longxin Qisheng (Suzhou) Biopharmaceutical Co., Ltd. The IND application for the AAV gene therapy product LX102 injection has been approved by the CDE for the treatment of wet age-related macular degeneration (wAMD).

The therapy is an in vivo gene therapy based on AAV vector, which can introduce the target gene expressing the anti-VEGF fusion protein into the patient's retinal cells, and the theory is that the last administration of the drug is effective for a long time. In previous IIT clinical studies, the therapy has shown good safety and efficacy.
12. GC301 of Beijing Jinlan Gene Technology
December 20, 2022 Beijing Jinlan Gene Technology Co., Ltd. IND, an AAV gene therapy drug GC301 adeno-associated virus injection, applied for CDE implied approval for early onset Pompeii disease (IOPD). On June 2, 2023, GC301 successfully held a phase I/II clinical trial initiation meeting in Peking Union Hospital of the Chinese Academy of Medical Sciences, marking the official launch of the clinical trial of GC301 injection for the treatment of infantile Pompeii disease in the main research center.
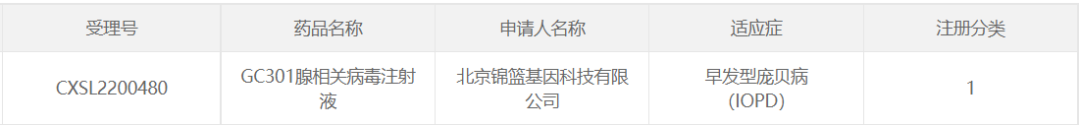
The therapeutic strategy is: After one-time intravenous infusion, the therapeutic gene can be widely expressed throughout the body to compensate for the GAA enzyme gene defects in liver, muscle, central nervous system and other tissues. GC301 has demonstrated good safety and efficacy in investigator-initiated clinical studies (Iits).
13. ZVS101e of Beijing Zhongyin Technology
December 6, 2022, Beijing Zhongyin Technology Co., Ltd. The IND application for "ZVS101e Injection" was approved by CDE for crystal-like retinal degeneration (carrying a CYP4V2 biallelic mutation). On February 20, 2023, the Phase I/II clinical trial of ZVS101e injection successfully completed the first subject enrollment and administration in the Eye Hospital of Tianjin Medical University.
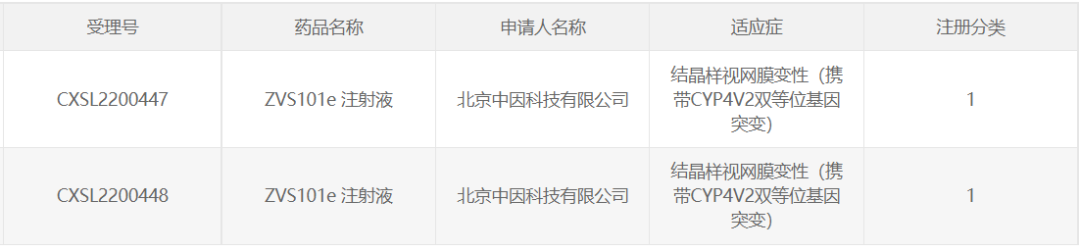
ZVS101e is a gene replacement therapy with AAV8 as the carrier, and its indications and treatment principle are consistent with Tianze Yuntai's VGR-R01, which was approved for clinical trials in November 2022.
14. GC304 of Beijing Jinlan Gene Technology
November 2022, Beijing Jinlan Gene Technology Co., Ltd. AAV gene therapy drug GC304 adeno-associated virus injection (" GC304 injection ") IND application for CDE implied approval for the treatment of hypertriglyceridemia in patients with recurrent acute pancreatitis.
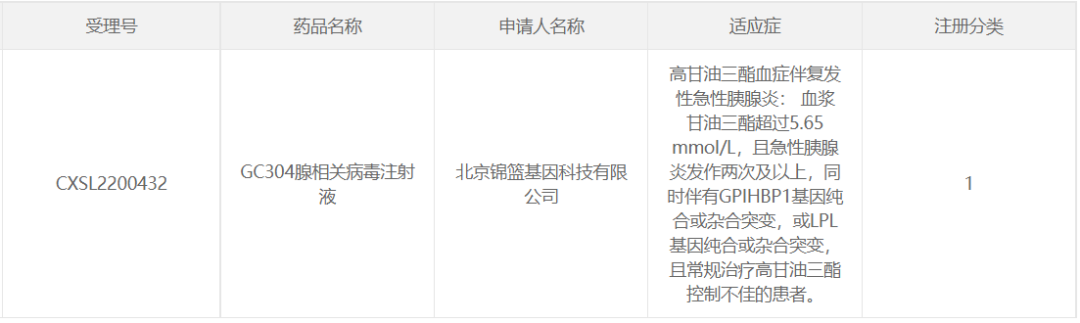
The therapeutic gene loaded with GC304 gene drugs is the lipoprotein lipase (LPL) gene, which is a key enzyme for hydrolyzing triglycerides (TG) in plasma lipoproteins.
15. KH631 of Chengdu Hongji Biotechnology
Chengdu Hongji Biotechnology Co., Ltd., November 15, 2022. Five IND applications for the AAV gene therapy drug "KH631 Eye Injection" were granted implied approval by the National Drug Administration for the treatment of neovascular (wet) age-related macular degeneration (wetAMD). In early May 2023, Professor Wei Wenbin's team of Beijing Tongren Hospital Affiliated to Capital Medical University completed the first patient administration of KH631 in Phase I clinical trial for the treatment of AMD.
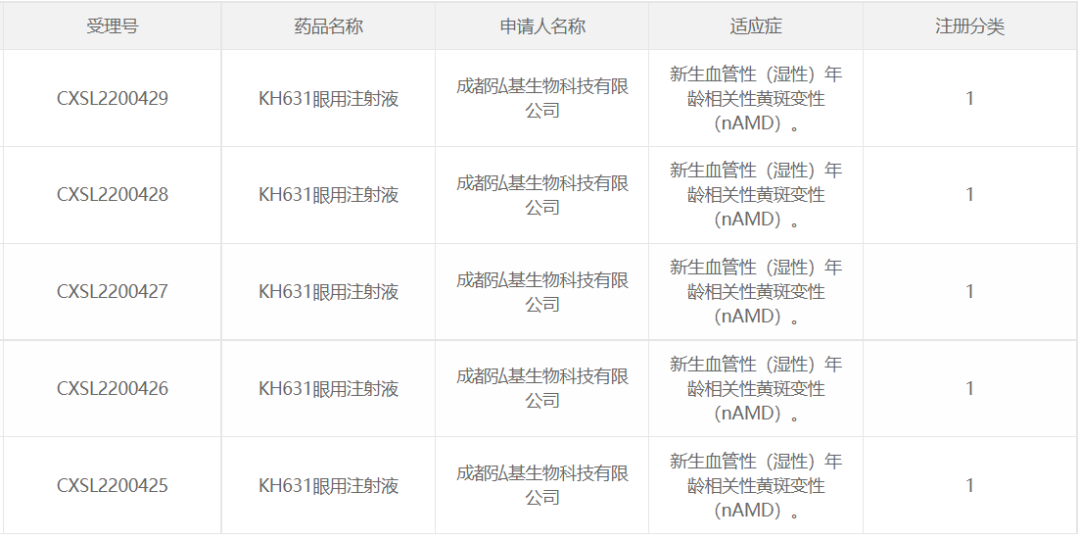
According to the relevant patent information disclosed by the company, KH631 achieves therapeutic effect through AAV overexpression of VEGF antagonists.
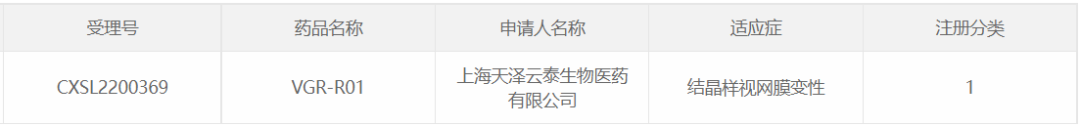
16. VGR-R01 of Shanghai Tianze Yuntai Biomedical
On November 1, 2022, Shanghai Tianze Yuntai Biomedical Co., Ltd. IND for the AAV gene therapy drug VGR-R01 injection for the indication of Bietti crystalline dystrophy (BCD) due to CYP4V2 gene mutation.
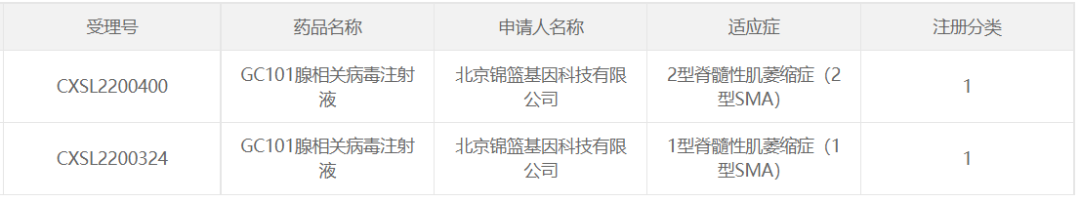
17/ GC101 of Beijing Jinlan Gene Technology
In October 2022, Beijing Jinlan Gene Technology Co., Ltd. The developed AAV gene therapy drug GC101 adeno-associated virus injection (" GC101 injection ") obtained the implied approval of CDE clinical trials for the clinical indication of type 1 spinal muscular atrophy (type 1 SMA), and on November 15 of the same year, the IND application for "GC101 injection" for the type 2 SMA indication obtained the implied approval of CDE.
18. FT-001 of Fangtuo Biotechnology
September 2022, Fangtuo Biotechnology (Suzhou) Co., Ltd. A clinical trial application (IND) for FT-001 injection, a gene therapy for RPE65 biallelic variant of inherited retinal degeneration, was granted implicit approval by CDE. On January 9, 2023, Fangtuo Bio-announced that FT-001 injection completed the first patient administration in Peking Union Medical College Hospital.

19. ZS801 of Sichuan Real&Best Biotech
On September 1, 2022, Sichuan Real&Best Biotech Co., Ltd. The clinical application of ZS801 injection was approved by CDE for the control and prevention of bleeding in men 18 years of age and older with severe and moderate to severe hemophilia B (congenital coagulation factor IX deficiency, coagulation factor IX < 2%).
 This is an AAV gene therapy, and the serotype of the AAV vector used in this therapy has a very low preexisting neutralizing antibody in the patient, which can cover more Chinese patients.
This is an AAV gene therapy, and the serotype of the AAV vector used in this therapy has a very low preexisting neutralizing antibody in the patient, which can cover more Chinese patients.
20. EXG001-307 of Shanghai Jiayin Biotechnology
Shanghai Jiayin Biotechnology Ltd., June 21, 2022. Announced that its self-developed AAV gene therapy EXG001-307 injection has received CDE approval for clinical trials for the treatment of spinal muscular atrophy type 1 (SMA type 1) with a biallelic mutation (deletion) of the surviving motor neuron 1 (SMN1) gene.

This is the first intravenous gene therapy product approved for type 1 SMA to enter a registered clinical trial in China. EXG001-307 is an AAV-based gene therapy that is expected to be effective for a long time with a single administration, and its principle of action and usage are similar to Novartis' Zolgensma. The innovative design for EXG001-307 is designed to reduce the side effects on the heart and liver, which is conducive to better use of its therapeutic effect.
21. VGB-R04 of Shanghai Tianze Yuntai Biomedical
In April 2022, Shanghai Tianze Yuntai Biomedical Co., Ltd. The first self-developed AAV gene therapy candidate, VGB-R04 injection, was approved for clinical trials for the treatment of hemophilia B caused by congenital coagulation factor IX deficiency.
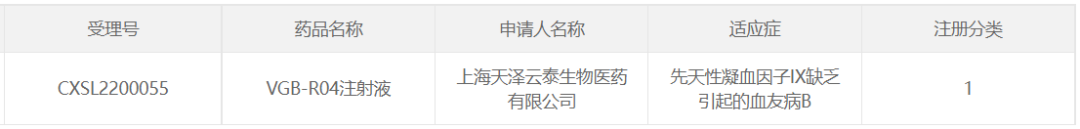
VGB-R04 is administered intravenously and delivered to the nucleus of the liver via AAV capsid mediated target gene (therapeutic gene) to express the protein hFIX Padua, a naturally occurring and highly active FIX variant (R338L), in liver cells. Its activity is about 8 times that of ordinary wild type FIX, which means that normal clotting function can be achieved at a lower expression level, thus reducing the dose of viral vector administration and improving the safety and efficacy of viral vector administration.
22. LX101 of Longxin Qisheng (Suzhou) Biopharmaceutical
In April 2022, Longxin Qisheng (Suzhou) Biopharmaceutical Co., Ltd. The self-developed AAV2-RPE65 gene therapy preparation LX101 eye injection has been approved for clinical trials for the treatment of RPE65 biallelic mutation-associated hereditary retinal degeneration (IRD) patients.
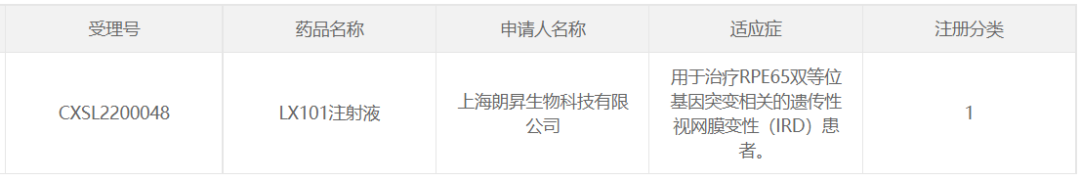
Gene therapy LX101 uses AAV2 as a carrier to introduce the RPE65 gene with normal function and optimized coding sequence into retinal cells in vivo to compensate for the loss of protein function caused by this gene mutation and restore vision. In previously conducted investigator-initiated clinical studies, the gene therapy has shown good safety and efficacy.
23. OAV101 of Novartis (Has entered the Phase III clinical)
In January 2022, Novartis (NYSE: NVS) submitted a clinical trial application for SMA gene therapy drug OAV101 injection (Zolgensma) in China to obtain clinical trial approval. On April 29, 2022, the relevant database showed that the therapy was launched in China for the first time, with 20 people enrolled in China, and the clinical trial was part of the China portion of the global Phase 3 clinical STEER study. On the afternoon of June 20, 2022, the Novartis Gene Therapy (OAV101) clinical trial China Research Center launch meeting was successfully held in Peking University First Hospital, the clinical trial is part of the global Phase III clinical STEER study in China, led by Professor Xiong Hui of Pediatrics at Peking University First Hospital. For patients aged 2 to 18 who are initially treated for type 2 spinal muscular atrophy (SMA).

Zolgensma is a gene replacement therapy for type 1 SMA that is theoretically effective for a long or even lifetime with one dose and is essentially a one-time treatment for the disease. This gene therapy uses scAAV9 vector to introduce normal SMN1 gene into the patient via intravenous infusion to produce normal SMN1 protein, thereby improving the function of affected cells such as motor neurons. In contrast, the SMA drugs Spinraza and Evrysdi require long-term repeated doses, with Spinraza administered by spinal injection every four months, while Evrysdi is a daily oral medication.
24. BBM-H901 of Shanghai Belief-Delivery (Has entered the Phase III clinical)
August 2021, Shanghai Belief-Delivery BioMed Co., Ltd. The gene therapy drug BBM-H901 injection independently developed for the treatment of hemophilia B was approved for clinical trial, and the clinical trial successfully completed the first subject administration at the end of December 2021, and has now entered clinical phase III, which is expected to be marketed in the second half of 2024 to the first half of 2025.

BBM-H901 is a gene therapy for intravenous administration of AAV. The target gene (therapeutic gene) carried by AAV can overexpress human clotting factor IX (hFIX), thereby improving and maintaining the level of clotting factor in the patient for a long time, which is suitable for preventing bleeding in adult male patients with hemophilia B. BBM-H901 is one of the earliest AAV gene therapy drugs to carry out clinical trials in China, and IIT clinical study (NCT04135300) has been started since 2019. The results of IIT clinical study show that this therapy has good safety and effectiveness, and the level of coagulation factor IX (hFIX) in subjects is significantly improved after administration. The level of hFIX in the blood was stable for a long time, and the annual bleeding rate of patients was significantly reduced, and no obvious adverse reactions occurred.
25、纽福斯的NR082(已进入III期临床)
2021年3月,纽福斯的NR082眼用注射液获得国家药品监督管理局颁发的注册性药物临床试验(IND)许可。2021年6月28日,纽福斯宣布,中国首个临床阶段的眼科体内AAV基因治疗药物NR082的I/II/III期第一阶段临床试验在中国完成首例患者入组及给药。2023年2月22日,纽福斯宣布,NR082眼用注射液用于治疗ND4突变引起的Leber遗传性视神经病变的III期临床试验在中国完成全部患者入组给药。
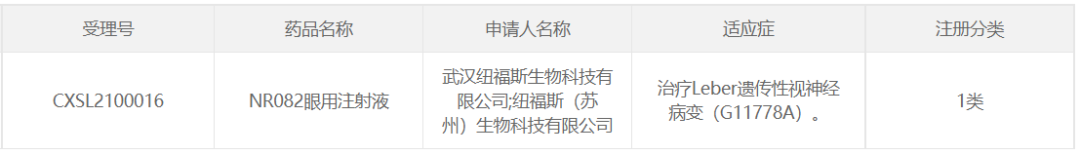
NR082 gene therapy drug uses AAV as a carrier for Leber hereditary optic neuropathy (LHON) caused by ND4 mitochondrial gene mutation. The drug delivery method is single intravitreal injection, and therapeutic genes are delivered to the damaged optic ganglion cells of patients through intravitreal injection to repair the biological respiratory chain of mitochondria. To restore the vitality and visual function of optic ganglion cells.
26. STSG-0002 of Staidson(Beijing) BioPharmaceuticals
In mid-June 2019, hepatitis B AAV gene therapy STSG-0002 was developed by Staidson(Beijing) BioPharmaceuticals Co., Ltd. Application for clinical trials, approved by the State Drug Administration for clinical trials of chronic hepatitis B treatment in mid-September 2019. In August 2020, the first subject was dosed.

STSG-0002 is a DNA gene therapy drug based on RNA interference technology using AAV as the carrier, developed by Sutazen, which has a significantly longer efficacy time than non-viral vector small nucleic acid RNA drugs, and is expected to be effective for a long time after single administration.
In addition, there are currently 7 AAV gene therapies on the market worldwide, as shown in the figure below:
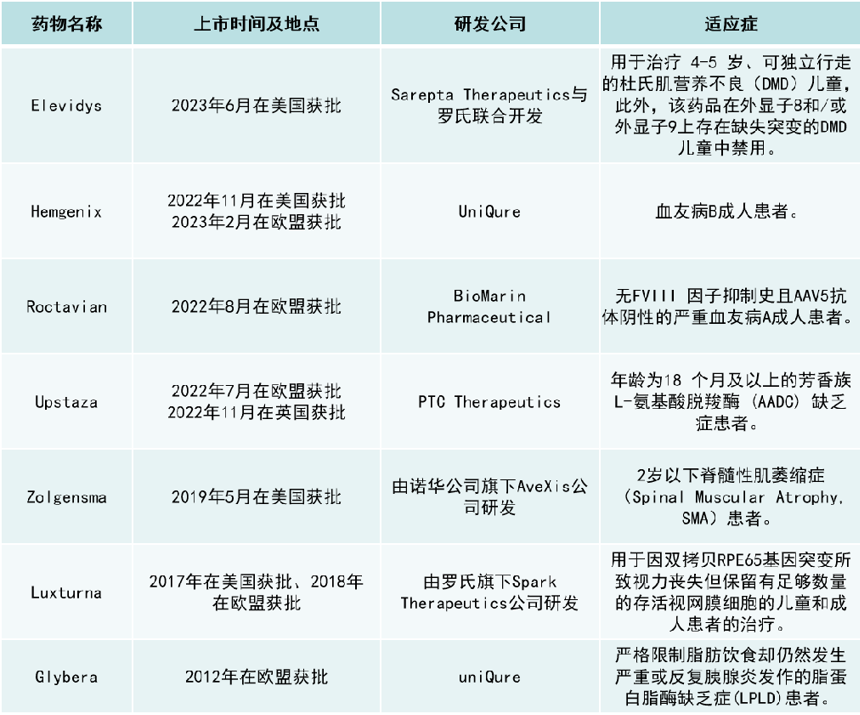
Among them, (1) Zolgensma was launched in May 2019, with sales of $361 million in the first year; $920 million in 2020; $1.351 billion in 2021; $1.37 billion in 2022. (2) Luxturna, achieved net sales of $55 million for the year ended September 2019 (2018-September 2019: $27 million and $28 million). After Roche announced the acquisition of Spark in October 2019, Luxturna's sales did not continue to be reflected in Roche's financial statements.