
Source: Changlink Health
Parkinson's disease (PD) is a very common neurodegenerative disease in the middle-aged and elderly groups, and the total number of Parkinson's patients in China has exceeded 3 million. Patients often present with slow movement, body tremors, postural disorders, and psychosensory disorders.
Around 10 million people worldwide are currently affected by Parkinson's disease, with the majority of patients concentrated in people over the age of 60. In China, about 1% of people over the age of 60 have Parkinson's disease, compared to 3-5% of people over the age of 70.
With the extension of people's average life expectancy, Parkinson's disease has become the third biggest "killer" faced by middle-aged and elderly people after tumor and cardiovascular and cerebrovascular diseases. The number of Parkinson's patients in China is expected to reach 5 million by 2030.
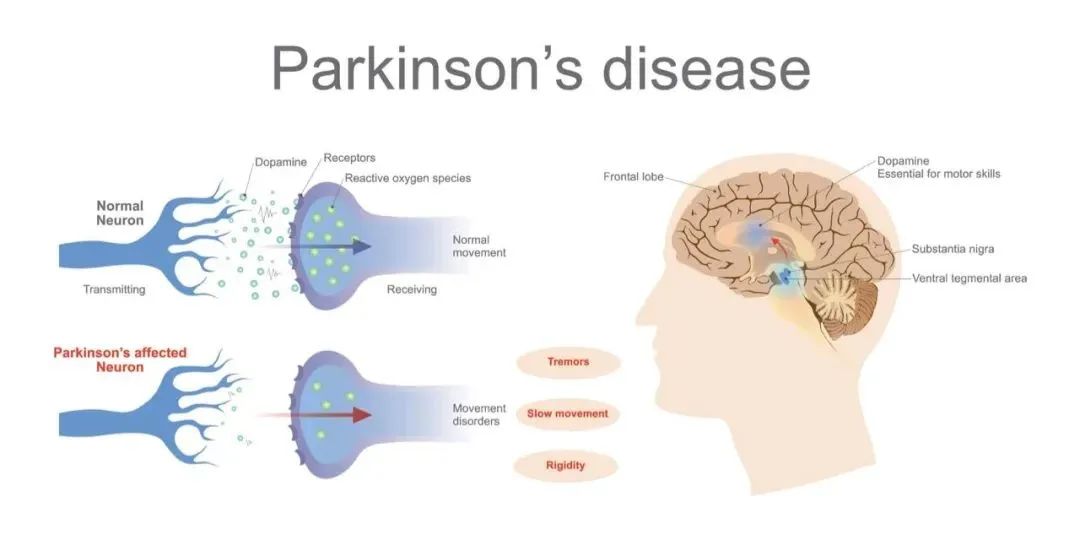
At present, the treatment of Parkinson's disease mainly focuses on two kinds of drugs and surgery. When patients are in the stage of strong symptoms, L-dopa can be used to relieve symptoms.
In certain neurons, L-dopa is converted into dopamine, which in turn alleviates the motor abnormalities caused by insufficient dopamine production in patients. However, the dosage of L-dopa is difficult to grasp, often causing side effects such as dyskinepsia and joint stiffness, and it is addictive, and most patients must take it for life.
Stem cell intervention in Parkinson's disease
Parkinson's disease affects people regardless of national boundaries and gender, and seriously affects people's lives and health. Once hailed as the world's greatest boxer, Ali was diagnosed with Parkinson's disease at the age of 42.
At present, Parkinson's disease is a disease that cannot be completely cured, supplemented by drug intervention, and surgical intervention represented by deep brain stimulation (DBS), etc., but traditional intervention means can not reverse neurodegeneration, can not increase the number of dopaminergic nerve cells. Therefore, it is of great significance to seek an intervention to increase the number of nervous system cells from the etiology.
With the rapid development of stem cell technology, the characteristics of stem cell multidirectional differentiation are gradually applied in clinical intervention of Parkinson's disease, which is a new hope for fundamental intervention of Parkinson's disease.

The main mechanism of stem cell transplantation intervention in Parkinson's disease is to use its secretion of neurotrophic factors, immune regulation and other neuroprotective functions to promote the repair of damaged dopaminergic neurons, or to induce its differentiation into dopaminergic neurons to replace damaged dopaminergic neurons in vivo and play a cell replacement role. There have been several clinical trials of stem cell intervention in Parkinson's disease around the world.
Clinical experiment of stem cell therapy for Parkinson's disease
Pluripotent stem cell therapy offers new hope for Parkinson's patients. In 1998, it was first reported that human embryonic stem cells (hESC) were isolated in vitro, and in 2007, it was first reported that human induced pluripotent stem cells (hiPSC) were prepared. Pluripotent stem cells are gradually being explored for the cell therapy of various diseases and injuries, and the cell therapy of Parkinson's disease has also entered the stem cell era.
Embryonic stem cell therapy for Parkinson's disease
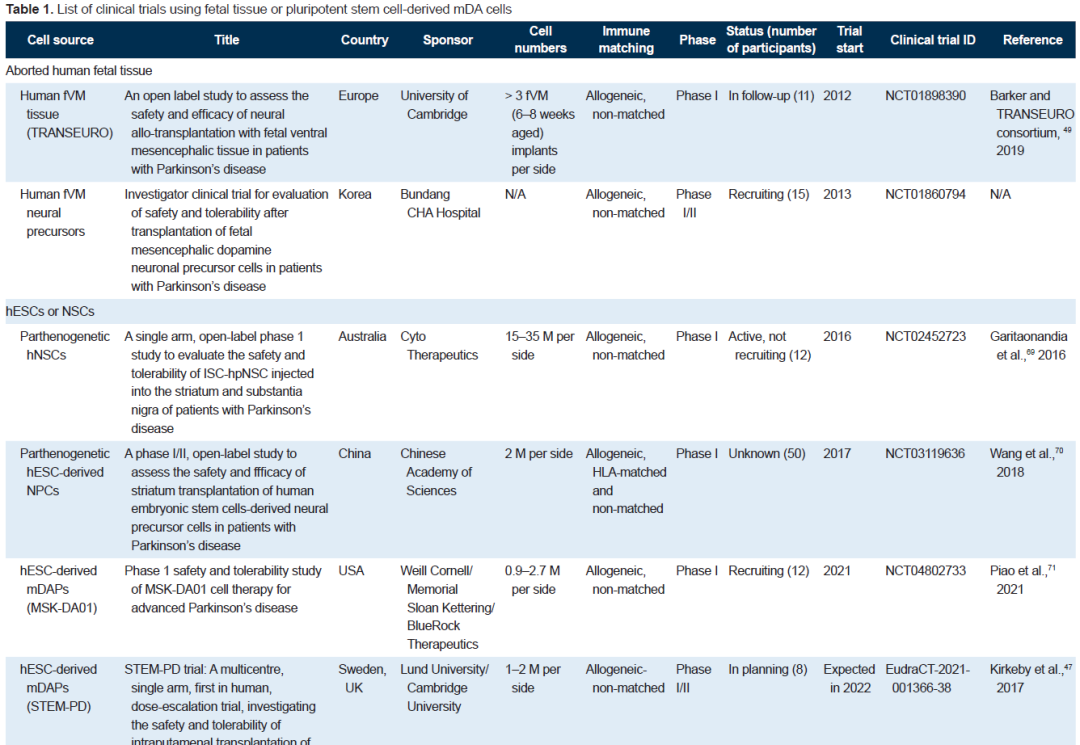
Embryonic stem cells are cells obtained from the Inner cell mass (ICM), which can differentiate into any of the three blastoderm cells, but cannot form the placenta, umbilical cord and other ancillary tissues. Three teams have conducted clinical trials internationally.
Among them, the team of Academician Zhou Qi of the Institute of Animal Science of the Chinese Academy of Sciences, using dopaminergic cells derived from human parthenogenetic embryonic stem cells (hpESC), proved the effectiveness and safety of dopaminergic cells in the Parkinson's monkey model, and opened clinical trials in 2017, which is also the world's first clinical study based on the use of embryonic stem cells to treat Parkinson's disease. In 2018, the journal Stem Cell Reports reported the results of Zhou's team's animal experiments.
In the primate model, the researchers transplanted clinical grade human parthenogenetic embryonic stem cells-derived midbrain dopaminergic cells into the brain of Parkinson's disease monkeys after strict quality inspection and followed up for two years. The results showed that the implanted cells could survive in the animals for a long time and further mature, significantly improving the symptoms of Parkinson's disease in monkeys, and no tumor formation was seen in 24 months.
HLA-iPSC treatment of Parkinson's disease
Induced pluripotent stem cells (IPscs) introduce inducible factors into mature somatic cells and reprogram them into pluripotent stem cells with characteristics similar to embryonic stem cells. Usually skin or blood is selected, fibroblasts or peripheral blood mononuclear cells are isolated, and reprogrammed to induce individual specific IPscs. HLA type identification must be performed before transplantation, and donor cells compatible with the patient's HLA must be selected for transplantation, which is one of the key factors for the success of stem cell transplantation.
According to different HLA phenotypes, scientists induced different IPscs and established HLA-IPSC libraries. Among them, the iPS Cell Research Institute (CiRA) of Kyoto University in Japan has established a clinical-grade iPSC haploid bank (HLA-homo iPS cell bank) covering nearly 40% of the Japanese population.
Autogenous iPSC therapy for Parkinson's disease
In essence, autologous IPscs are similar to HLA-IPscs. The difference is that autologous iPSC is made by reprogramming and directional induction differentiation of patient's (skin or blood) somatic cells, and can be used without or with little immunosuppressive agents.
In 2020, the New England Journal of Medicine (NEJM) reported that Kwang-Soo Kim's team at Harvard University in the United States used autologous IPSC-derived dopaminergic progenitor cells to treat patients with idiopathic Parkinson's disease. Two years of follow-up showed sustained improvements in motor function and quality of life, even without immunosuppressants.
Parkinson's stem cell clinical trials at a glance
The functional cure of Parkinson's disease has always been a difficult problem in the field of nerve regeneration. The results of clinical trials show that most stem cell transplants are safe, but the effectiveness is uneven, and the rapid development of stem cell technology makes regenerative medicine closer and closer to clinical transformation, and is expected to become the most promising alternative treatment strategy for Parkinson's disease.
Clinical trials of mesenchymal stem cells are being explored for optimal cell transplantation (including intravenous infusion, arterial infusion, intrathecal injection, striatal injection or nasal administration). Therapeutic mechanism: The efficacy of mesenchymal stem cells may depend mainly on their secretory properties rather than differentiation properties, and the engineered mesenchymal stem cells to enhance dopamine secretion is one direction.
Clinical trials of pluripotent stem cells are generally administered locally, with striatal injection or nasal administration at the transplant site. Mechanism of action: Cell replacement therapy increases dopamine concentration by transplanting mature, dopaminergic nerve cells. Clinical trials may use transplantation of dopamine progenitor cells derived from pluripotent stem cells (including hESC, HLA-iPSC, and autologous iPSC).
Stem cell therapy for Parkinson's disease is effective
In July 2018, the Japanese government approved a clinical trial at Kyoto University using induced pluripotent stem cells (iPS cells) to intervene in Parkinson's disease. On November 9, a Japanese scientific research team announced the injection of induced pluripotent stem cell neural progenitor cells into the brain of a 50-year-old Parkinson's disease patient, which is the world's first clinical trial of iPS cells to intervene in Parkinson's disease, the patient is recovering well, and the surgical effect and safety need to be long-term observation.
The domestic research on the intervention of stem cell transplantation in Parkinson's disease has also made gratifying achievements. As early as September 2011, the journal "China Tissue Engineering Research and Clinical Rehabilitation" published a research report entitled "Umbilical cord mesenchymal stem cell transplantation interferes with 8 cases of Parkinson's disease".
Eight patients with Parkinson's disease were admitted to stem cell transplant center of a Shanghai hospital. Since the 2nd week, umbilical cord mesenchymal stem cells were applied for carotid artery biopsy transplantation. Compared with before transplantation, the symptoms of tremor and tetanus of the patients were significantly improved. It is suggested that umbilical cord mesenchymal stem cell transplantation can improve clinical symptoms and quality of life of patients with Parkinson's disease to some extent.
A total of 19 patients with Parkinson's disease admitted to stem cell intervention center of a hospital in Lanzhou received umbilical cord mesenchymal stem cell intervention, including 8 males and 11 females, with a median age of 64 years and an average duration of disease of 4.5 years. Most of the patients experienced continuous exacerbation of symptoms after drug intervention such as levodopa and mdopa or continued drug intervention.
After lumbar transvaginal injection of mesenchymal stem cells, some symptoms of most patients were improved after intervention, especially abnormal symptoms caused by increased muscle tone were improved significantly. Muscle stiffness was reduced in sensitive patients after intervention, and stiffness, posture, gait, tremor and face were improved significantly.
In 2019, it was reported that they performed peripheral intravenous transplantation of human umbilical cord mesenchymal stem cells in 34 patients with Parkinson's disease. The results of the self-controlled before and after study of the included patients showed that the hUC-MSCs is safe and effective in Parkinson's patients through intravenous transplantation, and can improve the motor function and sleep quality of Parkinson's patients.
Compared with before transplantation, it was effective 1 month after transplantation and 3 months after transplantation. It was basically stable from 1 month to 3 months, and the curative effect could be maintained until the 3rd month. Gender and course of disease had influence on the curative effect, and female had more obvious curative effect on autonomic symptoms, cognitive function and daily living ability. RBD symptoms and RLS symptoms were more effective in patients with ≥5 years of disease course.
Compared with embryonic stem cells, induced pluripotent stem cells and other stem cells, mesenchymal stem cells have the advantages of wide source, easy sampling, low immunogenicity and no ethical controversy, and are regarded as the most ideal "seed" cells.
It can be seen that umbilical cord mesenchymal stem cells play a very positive role in the intervention of Parkinson's disease. Compared with traditional intervention methods, stem cell transplantation is a new and effective intervention method with broad prospects and provides new hope for the intervention of Parkinson's disease.