
Source: Stem cell says
Worldwide, 463 million adults (1 in 11) have diabetes, and this number is expected to reach 700 million by 2045. Diabetes kills 4.2 million people every year, and nearly 130 million people in our country have diabetes. Type 1 diabetes accounts for about 10 percent of diabetes cases worldwide, and type 2 diabetes accounts for about 90 percent. Lifestyle changes and medication are both effective ways to treat type 2 diabetes, and there have been many miracles.
On March 10, in the "Lifeline" column of CCTV12, Mother Yiming, director of the endocrinology department of the First Medical Center of the Chinese People's Liberation Army General Hospital, said that there has been a lot of research on stem cell treatment of diabetes. We have a clear understanding of how stem cells treat diabetes, and we will do better in the future.
-01-
Two strategies of mesenchymal stem cell therapy for diabetes
The role of mesenchymal stem cells is to regulate immune function, protect beta cells in patients, improve the islet environment, and thus reduce diabetes symptoms.
Mesenchymal Stem Cells (MSCs) can not only support and protect β cells, but also may be one of the sources of β cells. Mesenchymal stem cell transplantation is effective in patients with type 2 diabetes (T2D), but its efficacy in patients with type 1 diabetes (T1D) is controversial because of the poor ability of mesenchymal stem cells to differentiate into functional beta cells in vitro and the absence of transdifferentiation in vivo.
-02-
Mesenchymal stem cells for the treatment of diabetes
Mesenchymal stem cells are the most widely used stem cells in clinical trials. Mesenchymal stem cells can be obtained from adult tissue as well as from newborn tissue. It is generally believed that mesenchymal stem cells have three-line differentiation potential, that is, the ability to differentiate into osteoblasts (bone tissue), chondroblasts (cartilage), and adipocytes (adipose tissue) in vitro.
From a regulatory perspective, mesenchymal stem cells have been classified as advanced therapeutic products. Although mesenchymal stem cells are emerging as the most promising source of cells for "universal" cell therapy, their use in type 1 diabetes clinical trials remains highly controversial.
There are three different hypotheses about mesenchymal stem cell therapy for diabetes:
A)Functional β cells were obtained from islet precursor cells derived from mesenchymal stem cells.
B)Mesenchymal stem cells were used to generate β cells directly through in vivo transdifferentiation after transplantation.
C)Mesenchymal stem cells were used to support the survival of endogenous islets and induced to differentiate into islet precursor cells.
Until now, there has been a lack of strong evidence that mesenchymal stem cells can differentiate into functional beta cells or islet organoids in vivo and in vitro.
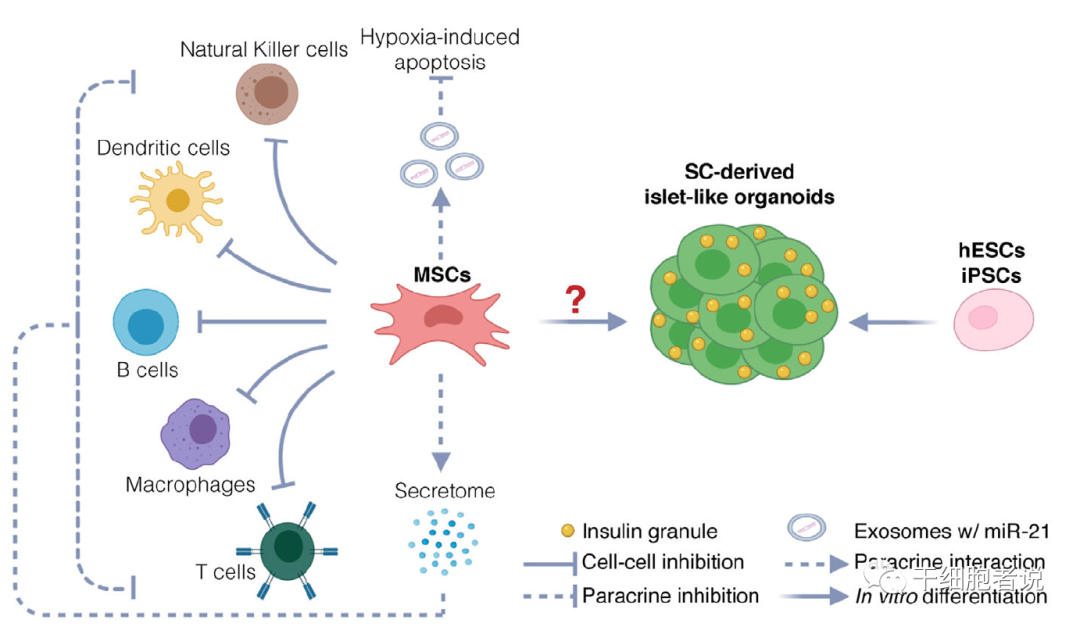
Potential therapeutic mechanisms of stem cell therapy for diabetes. Potential mechanisms include protection of endogenous islets and restoration of beta cells. MSCs protect endogenous beta cells by immunomodulating and inhibiting hypoxia-induced apoptosis. hESC and iPSC are also used to produce functional islet like organoids to restore beta cell function.
▉ Mechanism 1: Mesenchymal stem cells transform into beta cells (insufficient evidence)
Do mesenchymal stem cells differentiate into insulin-producing cells? Typically, the function of insulin-producing cells derived from mesenchymal stem cells is tested by stimulating insulin secretion in vitro and glucose tolerance in vivo in mice.
C-peptide is a by-product of insulin production and is derived from proinsulin along with insulin. C-peptide is often used as an indicator of insulin secretion in the body < If the indicator tested uses insulin instead of C-peptide, then the ability of beta cells to secrete insulin in response to glucose is often overestimated. Because the amount of insulin may not represent true insulin production, insulin is usually present in the medium as well.
One study showed a four-fold increase in C-peptide secretion in response to glucose stimulation in vitro, suggesting that mesenchymal stem cells may differentiate into insulin-producing cells capable of responding to glucose stimulation. Glucose tolerance tests showed that after transplantation into pancreatectomy or streptozotocin (STZ) -induced diabetic mice, although the results were not ideal, it still had some hypoglycemic ability. Kamalveni et al., using human umbilical cord blood mesenchymal stem cell (UCB-MSC) -derived islet generating cells for transplantation, detected low levels of human C-peptide 60 days after transplantation. Although human C-peptides could still be detected 150 days after transplantation, the range was highly variable (0.0-7.97pmol/ml) and was not therapeutic enough to maintain normal blood sugar levels in diabetic mice. This suggests that it is inefficient to induce mesenchymal stem cells to produce mature insulin-producing cells.
In summary, the evidence that mesenchymal stem cells can differentiate into beta cells is seriously insufficient.
▉ Mechanism 2: Mesenchymal stem cells improve islet microenvironment and immune regulation
On the other hand, preclinical studies conducted over the past 15 years support the protection of mesenchymal stem cells from islet grafts through two different mechanisms, namely, improving the cell survival environment and immune regulation.
Back in 2012, research by Ezquer et al. showed that injecting mouse mesenchymal stem cells into the veins of STZ-induced diabetic mice improved their blood sugar levels, reduced HBA1c to similar levels as non-diabetic mice, and increased total insulin levels. Fluorescence detection confirmed that mesenchymal stem cells could not differentiate into insulin-producing cells, but increased the expression of anti-inflammatory cytokines such as IL-13 and decreased the expression of pro-inflammatory cytokines (IL-1β, IL-18, TNF-α and MCP-1) through Treg cells.
The data suggest that part of the beneficial effect of mesenchymal stem cell therapy is achieved by reducing inflammation.
-03-
Nearly 2 years of clinical research based on mesenchymal stem cells
1. In October 2022, a research team from the Department of Endocrinology of Peking University Shenzhen Hospital published in the SCI journal "World journal of diabetes" entitled: Effectiveness and safety of human umbilical cord-mesenchymal stem cells for treating type 2 diabetes mellitus (Effectiveness and safety of human umbilical cord mesenchymal stem cells for the treatment of type 2 diabetes) research paper and suggest that human umbilical cord mesenchymal stem cell therapy may be a new treatment for type 2 diabetes.
Sixteen enrolled patients (age 18 to 70 years with a type 2 diabetes diagnosis and HbA1c HBA1c level ranging from 7% to 9.5%) received three umbilical cord mesenchymal stem cell transfusions, each one week apart, at a volume of 1×106/kg.
During the follow-up period, participants self-monitored fasting blood glucose and blood glucose 2 hours after meals seven times a week, while adjusting the dose of oral hypoglycemic agents and insulin according to the patients' blood glucose to maintain stable levels. All patients were medically evaluated again after 16 weeks.
The results suggest that the blood glucose index is significantly improved, the function of islet beta cells is improved, and the dosage of hypoglycemic drugs is reduced.

2. In 2023, the journal Stem Cell Translational Medicine published data from Professor Yoshiaki Mu's study evaluating the efficacy of UC-MSCs in the treatment of type 2 diabetes through retrospective continuous glucose monitoring.
In this randomized placebo-controlled trial, a total of 73 patients were assigned to receive 4 generations of UC-MSCs (n=37) or placebo (n=36) intravenously between October 2015 and December 2018, with each infusion number of 1×106/kg, once every 4 weeks, for a total of 3 infusions, and were followed for 48 weeks. Changes in TIR and glycosylated hemoglobin (HbA1c) were assessed at primary endpoints.
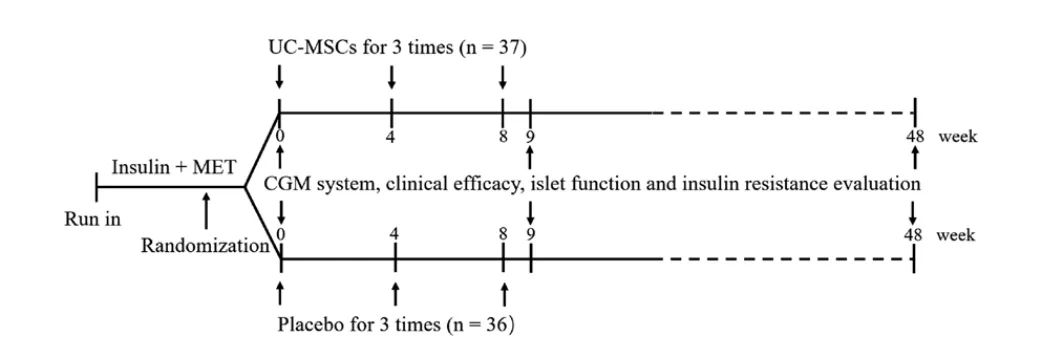
Study Design, MET, metformin; UC-MSC, umbilical cord derived mesenchymal stem cells; CGM, continuous blood glucose monitoring
Efficacy: This prospective, single-center, randomized, double-blind, placebo-controlled trial demonstrated that intravenous infusion of UC-MSCs increased TIR, reduced glycemic variability, reduced HbA1c levels, reduced daily insulin requirement, improved islet beta-cell function, and improved insulin resistance. AUCC-pep is an independent risk factor associated with T2D efficacy after receiving UC-MSCs intervention, and male patients are more likely to benefit from UC-MSCs treatment.
Three open-label clinical studies conducted by Liu X and Kong D initially confirmed the efficacy of UC-MSCs in the treatment of T2D. Consistent with these observations, this result also suggests that intravenous UC-MSCs reduced HbA1c and insulin consumption, underlining the efficacy of UC-MSC therapy in the treatment of T2D.
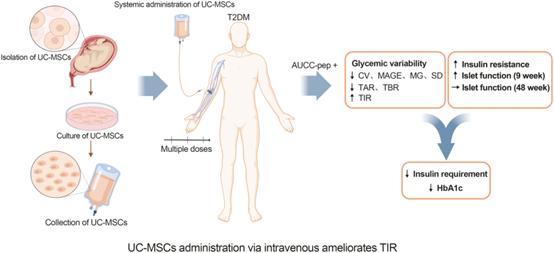
Safety: A female patient developed cerebral infarction 1 month after the 3rd UC-MSCs infusion. A male patient suffered an accidental femoral neck fracture 3 months after his third UC-MSCs infusion. In the judgment of the independent review Committee, these events are not considered to be related to UC-MSCs treatment.
-04-
Two-point thinking
一、Clinical trials of mesenchymal stem cells are usually administered intravenously. Therapeutic mechanism of action: Supporting islet health and survival in an indirect way (i.e. improving the islet microenvironment and regulating immunity), the beneficial effect is achieved by reducing inflammation. Clinical trials of mesenchymal stem cells in the treatment of type 2 diabetes can effectively reduce blood glucose level and exogenous insulin dose, but the efficacy has not reached the expectation and is not sustainable. So, is intravenous injection of mesenchymal stem cells an ideal treatment for type 2 diabetes?
二、After the new coronavirus, the number of people with abnormal blood sugar has increased, and according to many endocrinologists, the number of patients with abnormal blood sugar or diabetes will increase in 2023 (unofficial, no public data is available). Is it considered that the new coronavirus has caused certain impact on human immunity or endocrine and caused damage to pancreatic islets, such as causing damage after triggering inflammation and causing abnormal blood sugar?