Japan is one of the few countries in the world that allows stem cells to be used in clinical therapy. The diseases for which Japan's regenerative medical stem cells have been identified as effective interventions are: Vascular diseases (myocardial infarction, cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, renal insufficiency, arteriosclerosis, etc.), neurological diseases (spinal cord injury, cerebral infarction, Parkinson's disease, poliomyelitis, etc.), bone and cartilage diseases (osteoarthritis degeneration, chronic pain, rheumatoid arthritis, etc.), liver disease, lung disease, diabetes mellitus, asthma, refractory collagen diseases, immunity Disease, periodontal disease, etc., and has the function of preventing disease and anti-aging.
Mesenchymal Stem Cells (also known as Mesenchymal Stem Cells, hereinafter referred to as MSC) treatment belongs to the national "second type of regenerative medical technology" (to ensure the safety and legality of regenerative medical treatment). The Japanese government is very strict in the regulation of stem cell medical treatment, and only medical institutions approved by the Japanese government's Ministry of Health, Labor and Welfare (equivalent to the Chinese Ministry of Health) and the Certified Regenerative Medicine Committee can carry out stem cell transplantation medical treatment. The main stem cell treatment programs applied for and registered at the Ministry of Health and Welfare are summarized as follows: (The number of applications for each medical institution varies from applying for one type of treatment to applying for multiple treatments. Different medical institutions use slightly different words for the same treatment item, the author summarized as follows)
Treatments using autologous fat derived MSC via intravenous transfusion or local injection are:
[Treatment of spinal cord injury with autologous fat derived MSC];
[The use of autologous fat derived MSC in the treatment of cerebral infarction and cerebral infarction sequelae];
[Autologous adipose-derived MSC for amyotrophic lateral sclerosis (ALS), sickle cell disease (SCD), lewy body dementia (DLB), progressive ascending nuclear paralysis (PSP)];
[Autologous fat derived MSC therapy for heart failure];
[The use of autologous fat derived MSC in the treatment of chronic ulcer disease in severe lower limb ischemia];
[Use of autologous fat derived MSC in the treatment of chronic lung disease];
[Treatment of chronic kidney disease (CKD) with autologous fat derived MSC];
[Use of autologous fat derived MSC in the treatment of neurodegenerative diseases];
[The use of autologous fat derived MSC in the treatment of cirrhosis, liver fibrosis and liver dysfunction];
[Use of autologous fat derived MSC to treat joint pain];
[The use of autologous fat derived MSC in the treatment of knee degenerative diseases];
[Use of autologous fat derived MSC for chronic pain];
[The use of autologous fat derived MSC in the treatment of tendon and ligament injury];
[Use of autologous fat derived MSC in the treatment of motor organ function decline caused by exercise and aging];
[Autologous fat derived MSC therapy for osteoporosis];
[Autologous fat derived MSC therapy for arteriosclerosis];
[Use of autologous fat derived MSC in the treatment of cognitive dysfunction];
[Use of autologous fat derived MSC in the treatment of chronic diseases];
[Autologous fat derived MSC in the treatment of atopic dermatitis];
[The use of autologous fat derived MSC in the treatment of refractory skin diseases such as atopic dermatitis and scleroderma];
[Use of autologous fat derived MSC in the treatment of autoimmune diseases];
[ Use of autologous fat derived MSC in the treatment of lymphatic enlargement];
[Use of autologous fat derived MSC in the treatment of cerebrovascular diseases];
[Use of autologous fat derived MSC in the treatment of liver function diseases];
[Autologous fat derived MSC in the treatment of climacteric syndrome];
[Subcutaneous injection of MSC derived from autologous fat was used to treat pitting deformation of skin];
[Local injection of MSC from autologous fat to improve wrinkles and skin sagging due to aging];
[Treatment of diabetes with autologous fat derived MSC];
[Use of autologous fat derived MSC in the treatment of facial atrophy and skin aging];
[Use of autologous fat derived MSC for acne treatment];
[Scalp injection therapy with autologous fat source MSC for alopecia];
[The use of autologous fat derived MSC in the treatment of breast tissue defect];
[The use of autologous fat derived MSC in the treatment of bone resorptive dental diseases];
[Treatment of Alzheimer's disease with autologous fat derived MSC];
[Treatment of Parkinson's disease with autologous fat derived MSC];
Treatments using autologous bone marrow derived MSC include:
[Use of autologous bone marrow derived MSC in the treatment of central nervous system dysfunction];
[The use of autologous bone marrow derived MSC in the treatment of cerebral infarction sequelae];
[Use of autologous bone marrow derived MSC in the treatment of spinal cord injury];
Other MSC treatment programs using tissue sources other than the above are:
[Autologous pulp MSC was used to treat alveolar bone and jawbone];
[Using MSC derived from autocutaneous subcutaneous tissue for facial subcutaneous injection to improve wrinkles and skin sagging due to aging], etc。
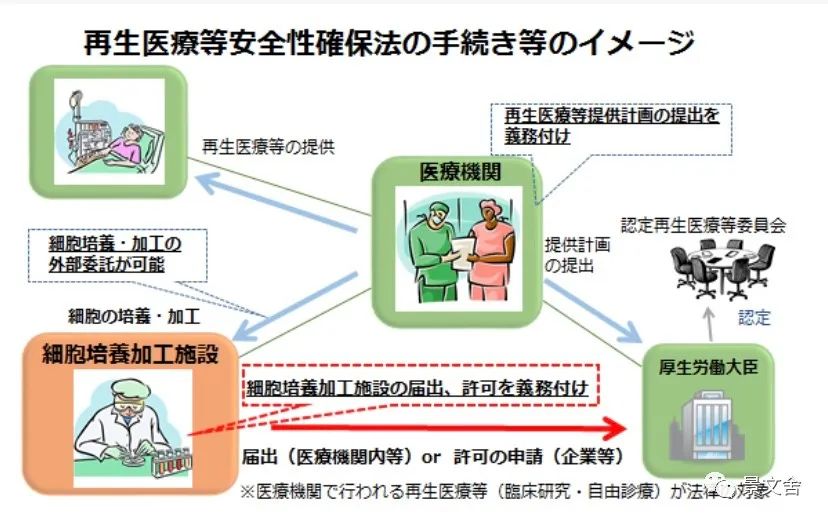
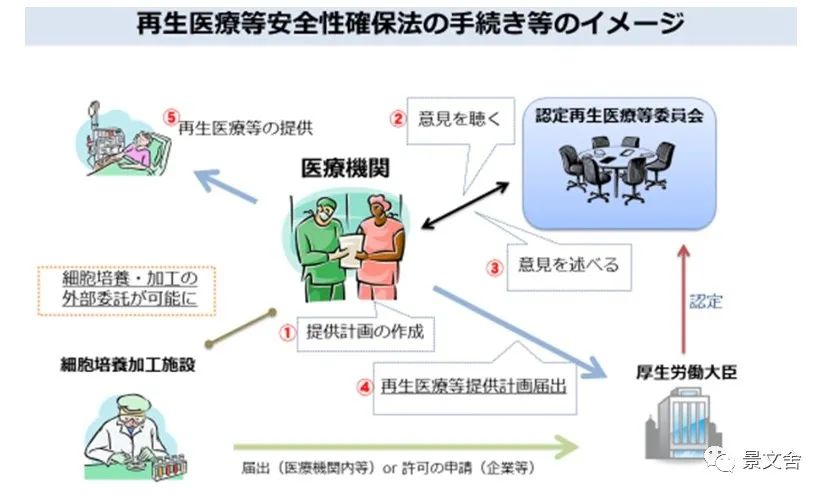
The following is the flow chart of the website of the Ministry of Health and Welfare of Japan on the application of medical institutions for the business qualification of stem cell therapy. The Japanese government is very strict on the regulation of stem cell medical behavior, and only medical institutions certified by the Ministry of Health and Welfare of the Japanese government and certified by the Regenerative Medicine Committee can carry out stem cell transplantation medical behavior.
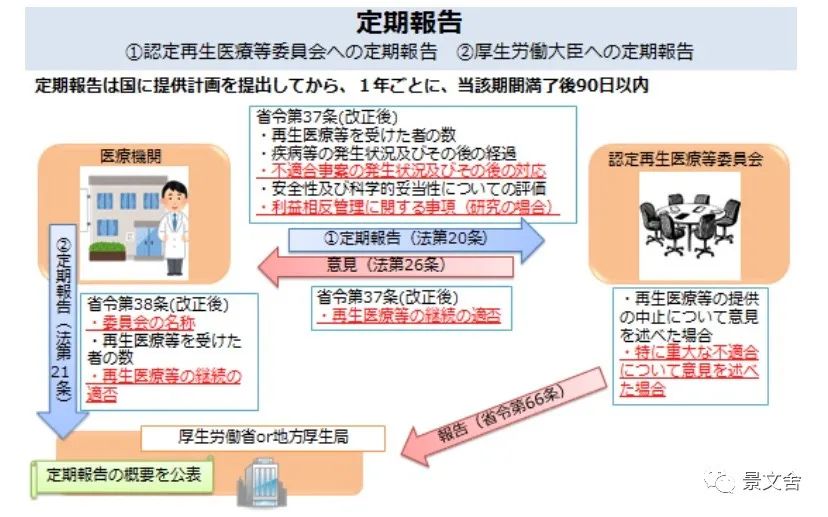
Approved stem cell treatment medical institutions are required to submit periodic reports to the Regenerative Medicine Board and the Ministry of Health and Welfare every year.
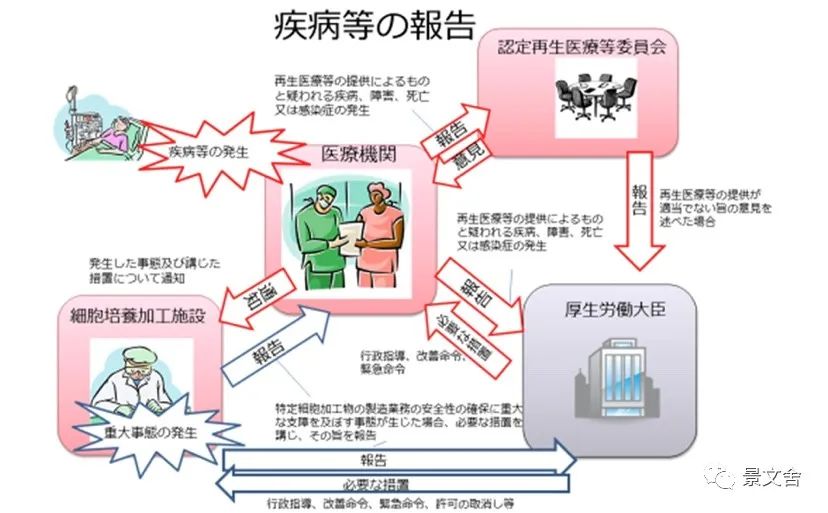
Any discrepancy with the submitted medical plan or patient's illness during the course of treatment must be reported immediately to the Regenerative Medicine Board and the Ministry of Health, Labour and Welfare.
"Cell culture processing facilities" that provide cell culture services to medical institutions for stem cell treatment must also obtain approval from the Ministry of Health and Welfare before they can operate.
The following picture: The website of the Ministry of Health and Welfare provides a flow chart for applying for the business qualification of "Cell culture processing facilities".