
Source: eMedClub
Recently, regenerative medicine company Cellusion announced that its IPSC-derived corneal endothelial Cell replacement therapy (CLS001) has been granted orphan drug designation (ODD) by the US FDA for the treatment of bullous keratopathy. This recognition will further accelerate the global development of CLS001.
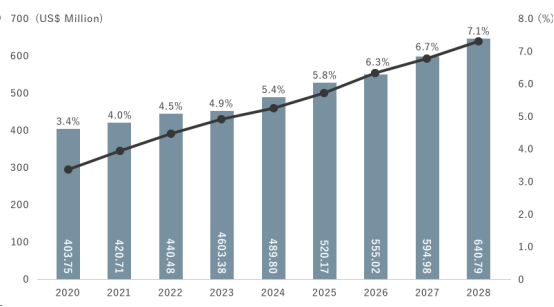
Bullous keratopathy is a blistering and visual impairment of the cornea caused by a reduction in the endothelial cells of the cornea due to cataracts, eye surgery or genetic factors. This progressive disease can lead to blindness in the absence of effective treatment, while the current standard treatment is through corneal transplantation, which replaces normally functioning corneal endothelial cells. According to public data, about 13 million people worldwide are waiting for corneal transplants, and only about 180,000 corneal transplants can be performed each year. The global corneal transplant market is forecast to grow from $421 million in 2021 to $641 million in 2028, with an estimated CAGR of 6.2%.
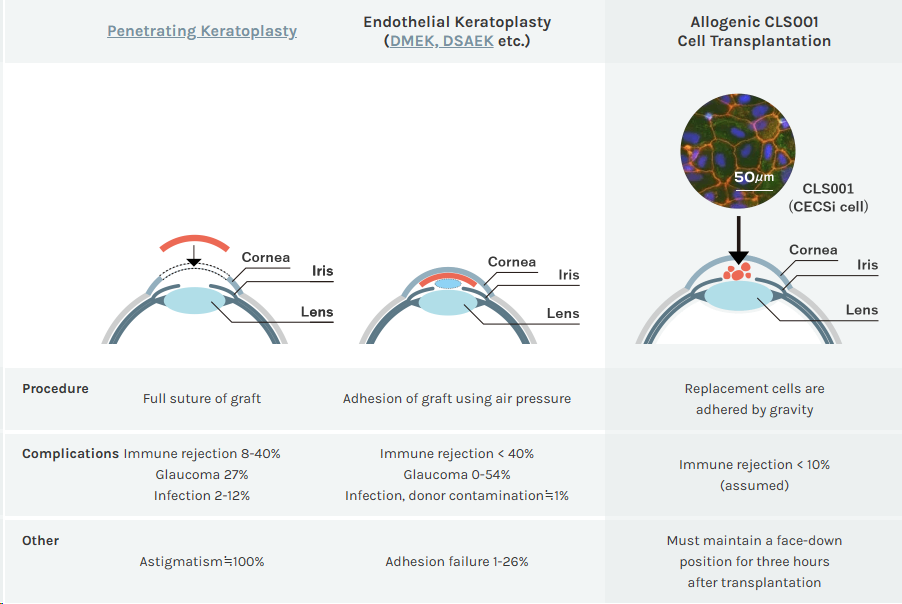
Based on Cellusion's proprietary technology, CLS001 is a CECSi cell for corneal endothelial regeneration that promises to change the current global shortage of corneal donors by combining "CECSi cells made from iPS cells with excellent proliferative properties" and "a simple injectable cell delivery procedure that requires no expertise." Unlike traditional corneal transplantation, which uses pneumatic adhesion, CLS001 therapy uses gravity adhesion, which must remain face-down for three hours after transplantation, resulting in smaller wounds and significantly reduced complications.
In addition, this product candidate addresses one of the current limitations of producing corneal endothelium-like cells from ESC or iPSC. Previous studies have shown that the process of producing corneal endothelioid cells from ESC or iPSC requires the development of neural ridge cells (NCC) as intermediates, which may produce a variety of cells including chondrocytes, fat cells, neurons, etc., which is not conducive to large-scale manufacturing. Cellusion's proprietary technology can differentiate iPS cells directly into corneal endothelium-like cells without the need for intermediates, which effectively ensures stable cell quality and facilitates rapid mass production of cells. In addition, CLS001 can be cryopreserved, which means that the corneal endothelial replacement cells needed for transplant therapy can be produced and stored in large quantities in advance, and they can be transported to different places so that they can be used when the patient needs them.
Previously, Cellusion announced that it had completed its first patient transplant of CLS001, and in a review by an independent data monitoring Committee held in January 2023, no adverse events were observed for 3 months after CLS001 transplant, and the patient's vision, central corneal thickness, and minimum corneal thickness had tended to improve. Currently, Cellusion is planning research in several regions around the world.
Cellusion was born in 2015 at the Department of Ophthalmology, Keio University School of Medicine, with a unique differentiation induction method that includes cells from iPSC to CECSi. In January 2022, Cellusion announced the completion of a 1.1 billion yen financing; In June this year, Cellusion completed a Series C financing of 2.83 billion yen, and as of the completion of the Series C financing, Cellusion has raised a total of 4.5 billion yen (about 226 million yuan), mainly to promote the phase 1/2 clinical trial of CLS001 in Japan and overseas.
September 2022, Cellusion entered into an exclusive license agreement with Hangzhou Xingsai Ruizhen to transfer license out, the exclusive rights to develop, manufacture and commercialize CLS001 in Greater China, including Chinese mainland, Hong Kong, Macau and Taiwan, to Xingsai Ruizhen, with the potential to receive more than US $100m, including advances, development and sales milestones, as well as gradient royalties.
Founded in July 2022, Hangzhou Xingsairuizhen Biotechnology Co., Ltd. is a platform company focusing on the research and development of innovative drugs in the field of cell therapy and regenerative medicine, focusing on the research and development and clinical transformation of stem cell-related technologies. It has completed a seed round financing of 45 million yuan, and the investors are Fosun Pharmaceutical's incubation fund Rehabilitation Capital New Drug Innovation Fund. According to public information, the company focuses on tissue regeneration and tumor therapy, and its tissue regeneration product candidates include corneal endothelial cell therapy introduced from Cellusion; In the field of tumor therapy, it is cooperating with top scientists in the field of tumor cell therapy in the United States to jointly develop stem cell-derived CAR-NK.
Recent progress in domestic iPSC field
iPSC is a promising track for applications in regenerative medicine, oncology, rare diseases and more. There has been a lot of progress on the iPSC circuit recently.
At the 65th Annual Meeting of the American Society of Hematology (ASH), Shenzhen Cell Inspire Biotechnology Co., Ltd. presented preclinical data on its IPSC-derived CAR-iNK product targeting GPRC5D. The purity of CAR NK cells derived from iPSC exceeded 99%, and the expression of NK cell activating receptors (NKG2D, NKp30) and co-stimulatory factors (CD226, CD244) exceeded 96%. In vitro experiments, single administration of CAR-iNK after resuscitation can significantly reduce tumor load in Xenograft model mice and improve the survival rate of mice. It is reported that based on this project, Sanqi Biology combined with its own gene editing platform advantages to further develop the first IPSC-sourced fourth-generation CAR-NK product CIB-315 for MM, and plans to declare IND in 2024.
In December, Wuhan Ruijian Pharmaceutical Technology Co., Ltd. "human dopaminergic precursor cell injection" obtained the implied approval of clinical trials for early-onset Parkinson's disease with the onset age earlier than 50 years old. At this point, Ruijian Medicine has carried out a strategic layout of full coverage of the life cycle and treatment cycle of Parkinson's disease.
In November, the world's first pipeline clinical grade iPSC-derived subtype of neuroprogenitor cell product for the treatment of ALS was granted the orphan drug title by the FDA, which is the world's first iPS derived cell drug with orphan drug status for the treatment of ALS.
In the same month, Beijing Chengnuo Medical Technology Co., Ltd. received implicit approval for its allogeneic endothelial Progenitor cells (EPCs) injection (ALF202) for the treatment of severe lower limb ischemia. It is based on iPSC induced differentiation of allogeneic endothelial progenitor cells (EPCs) injection. Zeno Medical also completed its first patient administration of ALF201 injection, an IPSC-derived cell candidate for the treatment of acute ischemic stroke.
In November, Anhui Zhongsheng Traceability Biotechnology Co., Ltd. "NCR300 injection" was approved for clinical use to prevent recurrence of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation, which is the second IND obtained by Zhongsheng Traceability in iNK cell therapy this year, further expanding the company's tumor product pipeline registered clinical trials.
Sum up
iPSC is progressing very fast in the world, especially in recent years, which can be regarded as a key node in the clinical transformation of research results. The domestic rise is also very rapid, many companies in this year to promote the candidate pipeline to the clinic. Although there are tumorigenicity, heterogeneity, immunogenicity and other challenges in the field of iPSC, from the data and research trends that have been disclosed, the current enterprises pay attention to its safety, and try their best to create technologies to reduce the proportion of undifferentiated IPscs while improving the efficacy. In the future, as iPSC product candidates increasingly show their potential in clinical trials, more players will be attracted to the game, and the industry chain will be further improved.