
Source: Pharmaceutical Proficient Bio
According to statistics, as of December 2023, more than 4,000 stem cell-related clinical projects have been registered worldwide, including 3,101 completed projects. It mainly involves autoimmune diseases (such as systemic lupus erythematosus), metabolic diseases (such as diabetes), respiratory diseases (such as COPD), osteoarthropathy (such as osteoarthritis, rheumatoid arthritis), reproductive system diseases (such as male erectile dysfunction), liver diseases, eye diseases and other fields.
Among them, China has more than 50 mesenchymal stem cell drug clinical trials, including diabetic foot ulcer, osteoarthritis, rheumatoid arthritis, pulmonary fibrosis, stroke, psoriasis, acute respiratory distress syndrome, liver failure, Crohn's disease, graft-versus-host disease and other diseases. With the increase in the number of clinical trials, the compound annual growth rate of China's stem cell market economy is expected to reach 16.5% by 2028.
PART1 Summary of recorded and marketed stem cell drugs
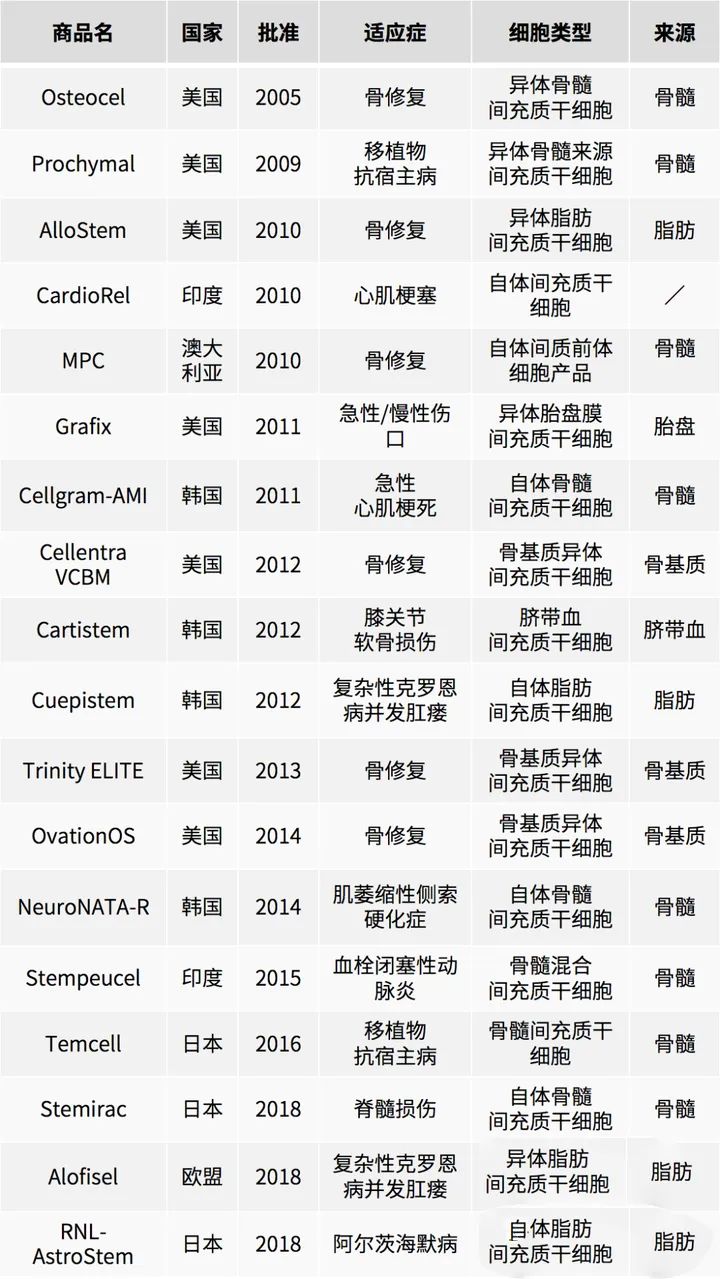
Mesenchymal stem cell drugs that have been marketed worldwide
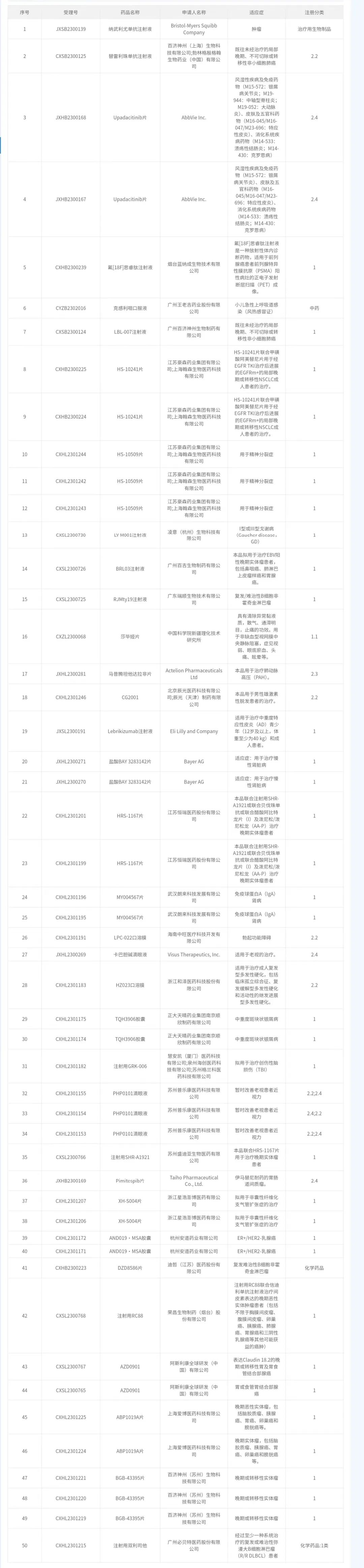
Summary of Implied Approval of mesenchymal stem cell Drug Clinical Trials in China (as of 2024.1.17)
PART2 Reference of new stem cell drug approval process
New drug development and listing is a complex process, in addition to preclinical research, clinical trials (phase I, phase II, phase III) these key steps, new drug IND application is also an essential link. Only through these stages, and each stage successfully through the approval, the new drug can finally obtain marketing authorization.
01New drug clinical research application
IND, full name Investigational New Drug Application, refers to the stem cell product through preclinical trials to the United States FDA (China is the "Drug Administration") submitted clinical research application for new drugs. If the FDA does not reject the application within 30 days of submission, then the product can proceed to clinical trials.
02New drug application
After the completion of all three phases of clinical trials, research data and data can be summarized and submitted to the FDA for a new drug application, that is, an NDA. The purpose of NDAs is to ensure the safety, effectiveness and quality control of marketed drugs. In general, the FDA should complete the NDA review within the prescribed time limit of 6 months. However, due to the large number of applications, most new drug applications take longer to be reviewed than this period.
PART3 Stem Cell regulatory policy
As an emerging technology, stem cell therapy requires strong regulation to ensure the health of patients and promote the healthy development of the entire industry.
In 2019, China issued the "Notice on Doing a good job in the supervision and Administration of Stem cell clinical research in 2019", which clarified that the clinical research and application specifications of stem cells should adopt a "dual-track management model", that is, it is jointly carried out by the "State Food and Drug Administration" and the "Health Commission" to establish stem cell technical specifications in line with China's national conditions.

From 2022 to 2023, China has successively promulgated a number of regulations such as "Guidelines for the Production Quality Management of Cell Therapy Products (Trial)", "Guidelines for the Application of key 2022 Projects of" Stem Cell Research and Organ Repair "(draft for comment), and" Technical Guidelines for clinical Trials of human stem cells and their derived cell therapy Products (Trial) ". More standardized management of clinical trials, quality management, production management, and clinical applications of stem cell products (such as stem cells and organ aging, novel coronavirus pneumonia, cardiovascular function remodeling) has been carried out.

PART4 Summary
In 2023, the global stem cell therapy market revenue is about 286 million US dollars, and has broad development potential. The stem cell market is expected to be valued at $615 million by 2028. At present, the approved stem cell products in the world are mainly concentrated in Japan, South Korea, the United States, the European Union and other places. In recent years, China is also vigorously supporting the development of stem cell technology, and the development of stem cells has been included in the "14th Five-Year" development plan.
I believe that in the near future, stem cells will be like "smart phones", deeply affecting each of our lives. We also look forward to the continuous expansion of stem cell drug research scale, regenerative medical technology can benefit more patients!