
With the rapid development of medical science and technology, stem cell therapy has become one of the most advanced treatment methods.
As a representative emerging technology in the field of regenerative medicine, stem cell therapy plays an important role in organ repair and tissue regeneration, and has broad application prospects. According to Guangming Daily, "Eight countries, including Australia, New Zealand and the United States, have conducted expanded trials of stem cell therapy technology. This technology has been used for 130 indications, that is, 130 diseases that have not been treated with drugs or have little effect, and can be treated with stem cells." 2023, stem cell research and development has also made a number of breakthroughs!
Stem cell therapy
Stem cells are a kind of cells with self-renewal and multidirectional differentiation potential, and can differentiate into multiple functional cells under certain conditions. Stem cell therapy is a method of using the differentiation potential of stem cells to treat disease. Transplantation of healthy stem cells or stem cell exosomes into patients can replace or repair damaged cells or tissues, thus achieving the purpose of treating diseases.
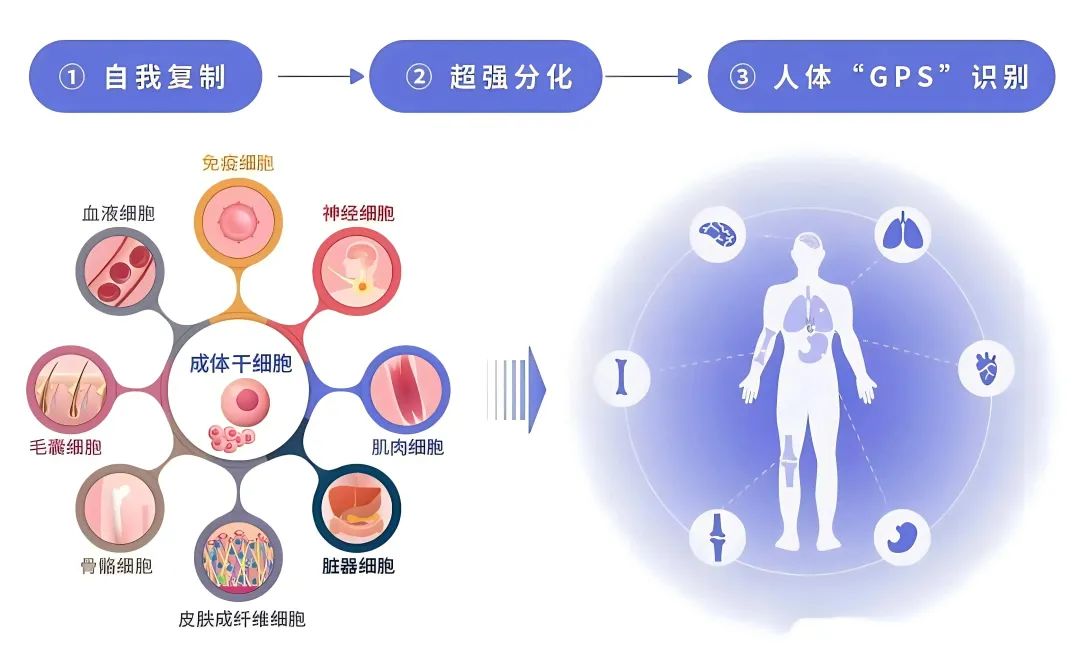
Breakthrough in stem cell treatment of chronic and refractory diseases
Stem cells are known as the raw materials of life. Various types of stem cells, including hematopoietic stem cells, mesenchymal stem cells, and induced pluripotent stem cells, have been used in clinical trials and treatments. And stem cell therapy is constantly expanding its therapeutic fields, including blood system diseases, nervous system diseases, digestive system diseases, cardiovascular system diseases, respiratory system diseases, skeletal system diseases, endocrine system diseases, immune system diseases, urinary and reproductive system diseases, as well as anti-aging, enhance immunity and other aspects.
Diabetes
Diabetes mellitus is a group of lifelong metabolic diseases characterized by chronic hyperglycemia caused by multiple causes.
On March 9, 2023, VX-264, a new stem cell therapy developed by Fortey Pharmaceuticals, was approved for an investigational New Drug Application (IND) by the U.S. Food and Drug Administration (FDA) to treat type 1 diabetes. Prior to this, another stem cell product, VX-880, also developed by Fortey Pharmaceuticals, was announced at the 83rd Scientific Sessions of the American Diabetes Association. VX-880 replaces damaged beta cells with healthy cells created from stem cells. After receiving VX-880, all 6 patients produced endogenous insulin (C-peptide) and improved blood glucose control, but they needed to be combined with immunosuppressants.
On June 28, 2023, CellTrans's cell therapy drug "Lantidra" was approved by the U.S. Food and Drug Administration (FDA) and is the first allogenic islet cell therapy made from donor-derived islet cells for the treatment of type 1 diabetes. The marketing of Lantidra is based on two non-randomized clinical trials in which 21 of the 30 patients enrolled were off insulin for at least one year, 11 were off insulin for one to five years, and 10 were off insulin for more than five years.
Recently, the plastic surgery department of the Affiliated Hospital of Guangdong Medical University successfully applied mesenchymal stem cells to treat the intractable wounds of diabetic feet, and the patients achieved remarkable results. The "Randomized, open and parallel controlled clinical research project on the application of human umbilical cord mesenchymal stem cells in the treatment of intractable wounds of diabetic foot" of the Orthopedic Surgery Department of the Affiliated Hospital of Guangdong Medical University was approved by the National Health Commission on October 20, 2021, becoming the first batch of approved clinical research projects on the treatment of diabetic foot with stem cells. It is also the first successful clinical research project for stem cell treatment of major diseases in the field of plastic surgery in China.

Multiple sclerosis
Multiple Sclerosis (MS) is a neurodegenerative disease, due to the immune system mistakenly attacks and destroys the myelin sheath, involving the optic nerve, spinal cord, brain stem, periventricular white matter and other parts of the brain, resulting in abnormal vision, limb movement disorders and ataxia and other clinical symptoms. Patients gradually lose sensation and function in their limbs and other parts of the body.
In 2023, a team of researchers at the University of Cambridge in the United Kingdom showed in preclinical studies that reprogramming skin cells into brain stem cells and transplanting them into the central nervous system could help reduce inflammation while helping to repair damage caused by multiple sclerosis. This was followed by the publication of early clinical trial results in the journal Cell Stem Cell.
The results showed no increase in disability and no significant deterioration in cognitive function or symptoms during the 12-month follow-up period. The results showed that the higher the stem cell dose, the smaller the reduction in brain volume over time, which may be related to the effect that stem cells have on suppressing inflammation. SCs are considered a promising treatment for neurodegenerative diseases such as mesenchymal stem cells (MSCs). The latest advances have raised expectations that stem cell therapy may help ameliorate this damage.

Parkinsonism
Parkinson's disease (PD) is the most common degenerative disease of the central nervous system in middle-aged and elderly people, with movement disorders, tremor and muscle rigidity as the main manifestations. Surgical and drug treatment has many complications and the curative effect is not lasting, which brings great burden to patients and their families.
In February 2023, a new stem cell transplant therapy developed by Lund University in Sweden was successfully transplanted in a patient, marking a milestone in the treatment of Parkinson's disease (PD).
In August 2023, Huixin Medical Valley, an innovative biomedical company focusing on the field of cell therapy for nervous system diseases, announced its first pipeline: autologous iNSC-DAP(dopaminergic precursor cells that induce neural stem cell differentiation) preparation, which has completed autologous transplantation of stem cells related to Parkinson's disease treatment in Xuanwu Hospital of Capital Medical University. This experiment is the first clinical study of Parkinson's disease treatment using iNSC as seed cells in the world, marking that China has entered the forefront of the exploration of stem cell therapy for neurological diseases.
In November 2023, Biostar, a Korean adult stem cell research institute, obtained the approval of the Japanese Ministry of Health, Labor and Welfare for its regenerative medicine technology to treat Parkinson's disease using autologous fat stem cells cultured with patented technology, and began treatment at the Shinjuku Clinic in Tokyo in December. The approved stem cell treatment includes intravenous injections of 150 million to 250 million adipose-derived stem cells and 50 million cells injected into the spinal cord cavity five times every two to four weeks. The combination of intravenous and spinal cord delivery is expected to improve the effectiveness of the treatment, which may also be effective for other incurable neurological disorders.

Heart failure
Heart Failure (Heart Failure) refers to the syndrome mainly caused by circulatory dysfunction due to myocardial diastolic and/or systolic dysfunction in the case of appropriate venous return, and the cardiac output is not enough to maintain the metabolic needs of tissues. Clinically, it is characterized by reduced cardiac output, reduced tissue hemoperculation, and venous congestion of pulmonary circulation and/or systemic circulation, so it is also called congestive heart failure or cardiac insufficiency.
In June 2023, the newly published Chinese Expert Consensus on Autologous stem Cell Transplantation for the Treatment of Heart Failure (2022) in China proposed that stem cell transplantation is expected to become a treatment method with more potential and application prospects for the treatment of heart failure.
In September 2023, Japan was the first in the world to carry out a treatment trial of transplanting "cardiac muscle balls" into patients with severe heart failure, and confirmed that the symptoms of two patients who underwent surgery were significantly improved. The "heart muscle balls" are processed from heart muscle cells made from artificial pluripotent stem cells (iPS cells). After the surgery, the function of the patient's heart, which had hardened and become difficult to contract due to myocardial infarction, doubled. This is the result of a new phase of work using iPS cells to treat heart failure, which is scheduled to be used in 2025.
Muscular dystrophy
Muscular dystrophy includes a variety of diseases that cause muscle loss, and in the most severe cases, patients gradually become unable to walk or even breathe on their own, eventually dying.
In May 2023, Professor Hidetoshi Sakurai and his team at the iPSC Research and Application Center (CiRA) at Kyoto University developed a novel GMO-free differentiation protocol, Human IPscs can be differentiated into fetal MuSCs with higher regenerative potential than iPAX7(a muscle stem cell with strong PAX7 expression) muscle progenitor cells generated by the transgenic regimen.
In differentiating human IPscs into fetal MuSCs, the researchers found that monitoring the expression of a gene called MYF5 could help determine whether IPscs had high regenerative potential - cells expressing MYF5 showed strong regenerative potential. The researchers also found that the duration of MYF5 gene expression in iPSC was positively correlated with PAX7 expression levels. For this study, in a cell therapy targeting DMD, the researchers injected muscle stem cells with intact anti-muscular dystrophin genes into mice, and the transplanted cells regenerated normal muscle.
Small series summary
At present, there are a number of foreign products approved for listing, as of December 2023, more than 60 new stem cell drugs have been approved by the National Drug Administration (NMPA) for clinical trial research, and Hong Kong, Shenyang, Shenzhen, Shandong, Beijing, Tianjin, Shanghai and other places are also the first and then the stem cell support policy landing. With the deepening of research, the future stem cells are expected to conquer more diseases that are currently difficult to cure, let us look forward to stem cell therapy to bring the Gospel to more patients!