
In 2006, Shinya Yamanaka successfully prepared embryonic stem cell-like cells by introducing Oct3/4, Sox2, c-Myc and KLF4 into somatic cells. According to their pluripotency, he named them "induced pluripotent stem cells" (iPSC). In fact, iPSC can differentiate into different cell types by growing under different culture conditions. IPSC has proven to have great promise, including the ability to address the ethical and safety issues of the use of embryonic stem cells. IPSC is used in a variety of scientific areas, such as disease modeling and drug discovery, developmental biology and regenerative medicine. The adoption of CRISPR-Cas9 technology can quickly and accurately edit the gene of iPSC, accelerate and improve these applications.
CRISPR-iPSC-based disease modeling removes the main obstacle to obtaining disease tissue samples for traditional disease modeling applications (exposing patients to dangerous biopsies). IPSC can be treated with an ideal combination of growth factors and culture conditions to differentiate into specified cell types. In this way, CRISPR-based technology can accelerate the development of neurodegenerative diseases, which uses iPSC to simulate the entire mutant gene pool that leads to the development of neurodegenerative diseases. Therefore, the genome-edited disease model based on iPSC can be used to generate useful information about disease pathophysiology and its potential drug targets, thus opening the door for the search for new treatments. Neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis.
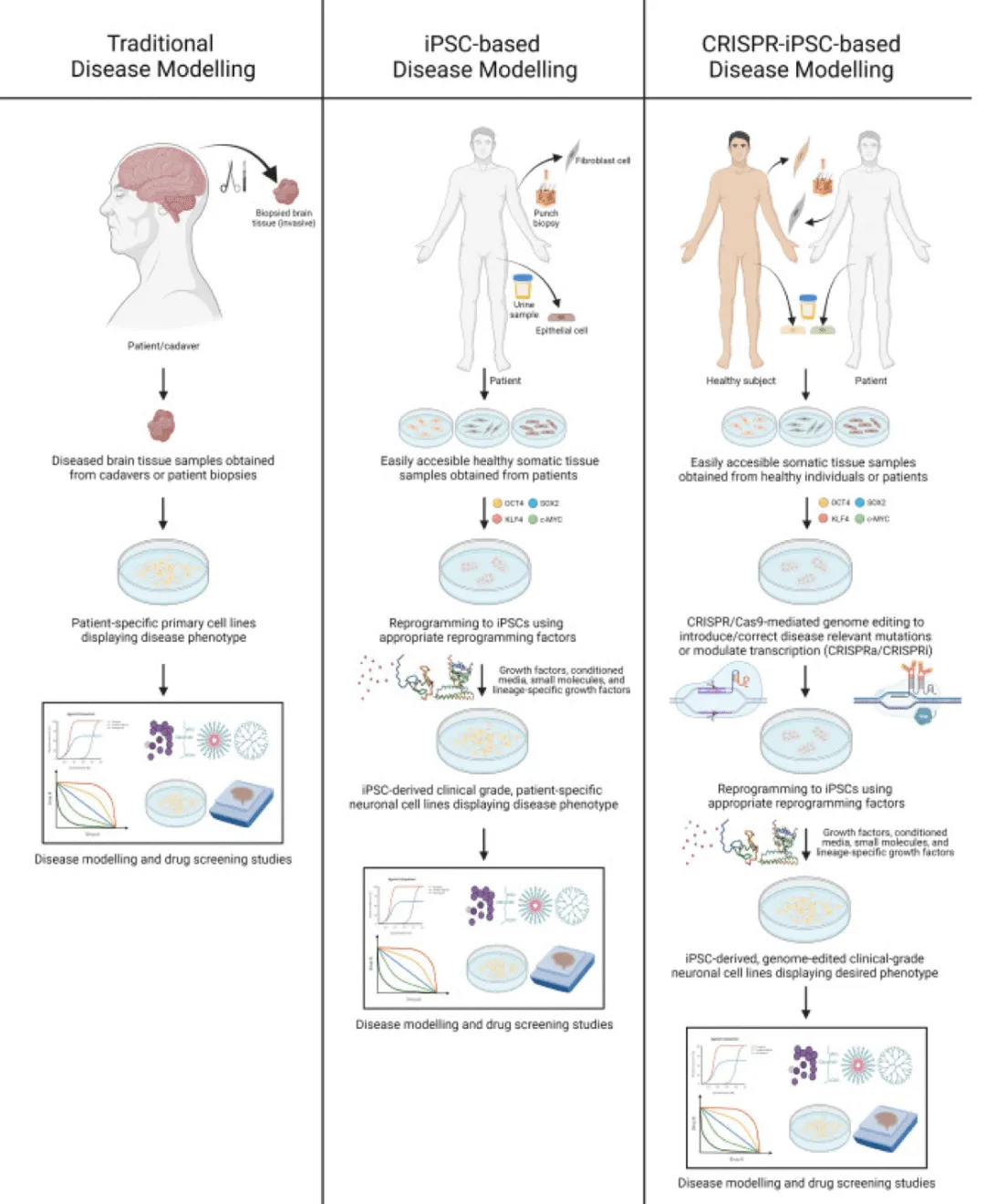
Disease Modeling based on CRISPR-iPSC
The CRISPR-Cas9 system has the potential to transform developmental biology by giving researchers unparalleled insight into the interaction between genome activity and developmental processes such as cell division, proliferation and morphogenesis in many animals. In addition, CRISPR screening in iPSC has also been used to study neurodevelopmental and degenerative diseases, cell fate norms and survival mechanisms of specific cell lineages. For example, single-cell CRISPR screening has been successfully used to identify cell fate regulators during brain development in human nervous organs produced by iPSC.
IPSC introduces innovative development of regenerative medicine in this area to restore damaged tissue by providing potentially unique treatment options. The combination of iPSC and CRISPR-Cas9 technology with tissue engineering technology may significantly expand the field of regeneration research. For example, CRISPR-Cas9 can be used to correct a single genetic anomaly associated with a variety of diseases, including HCM and DCM. Then use iPSC to create a corrected mutated cell line. Last but not least, tissue engineering can be used to create healthy tissue from iPSC and then transplant it into patients.