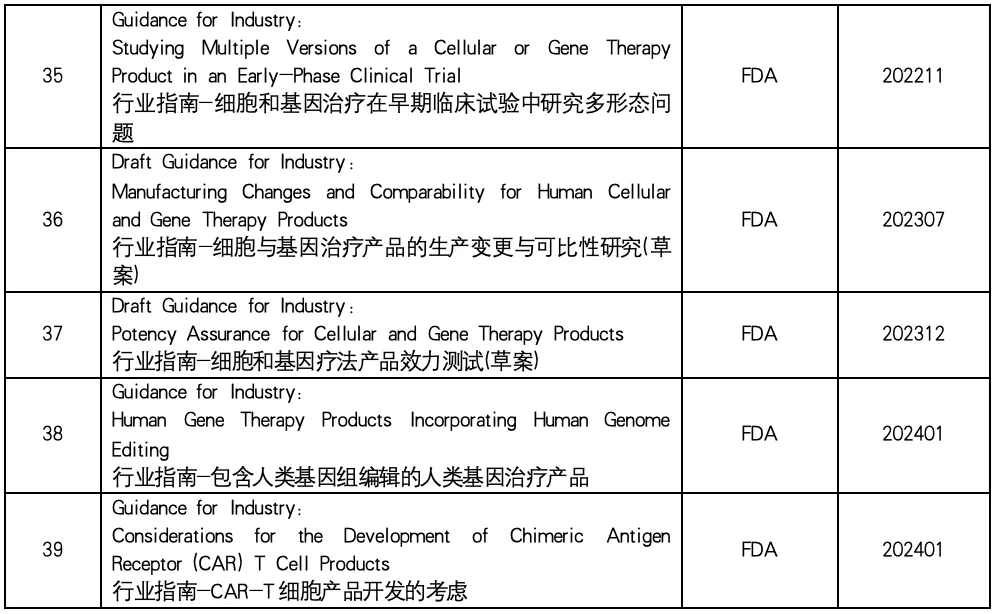
中

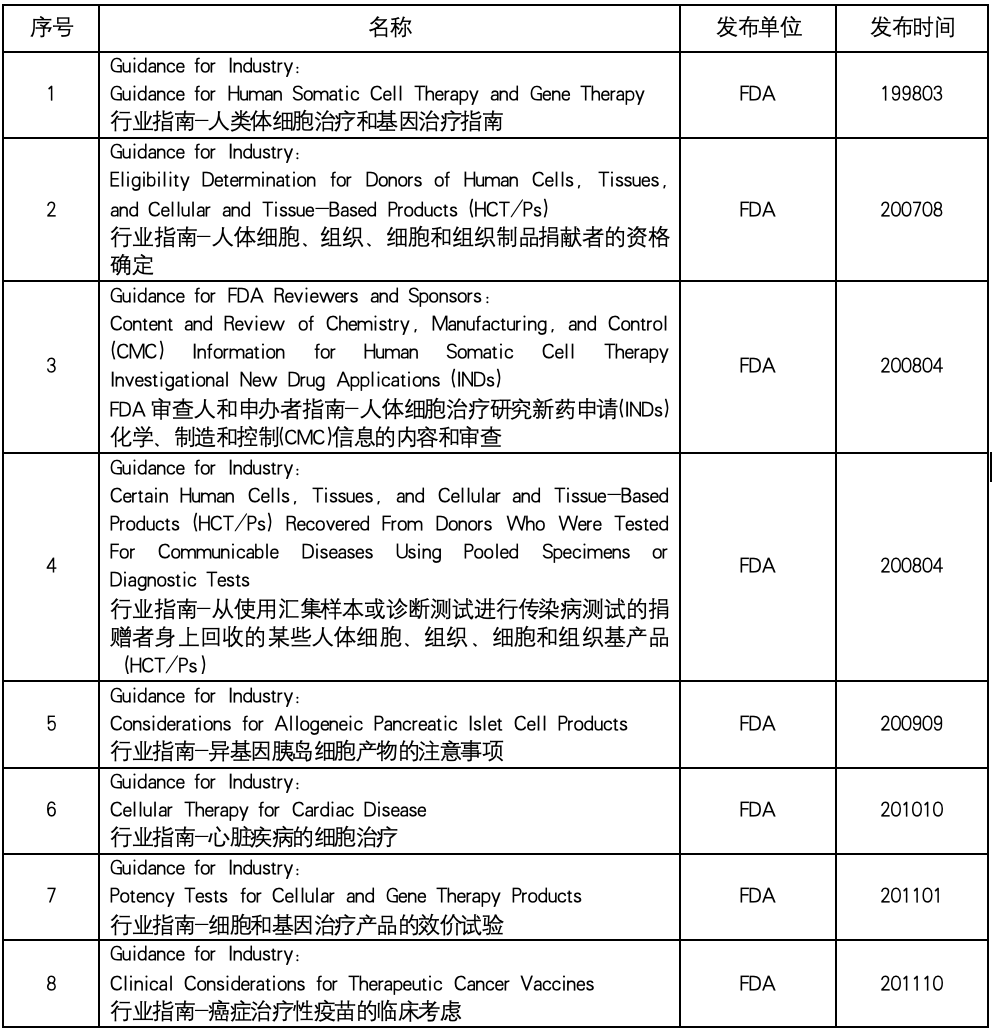
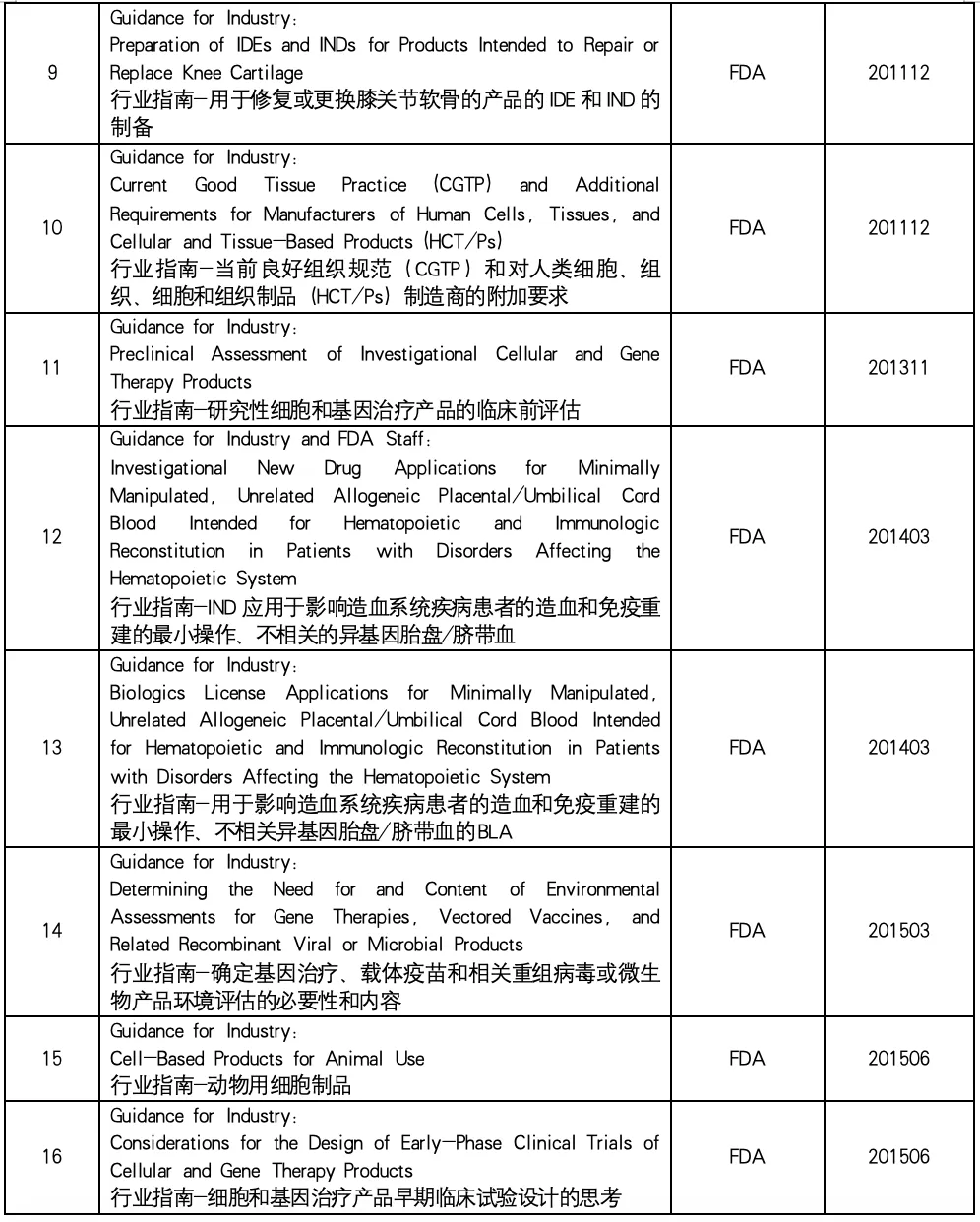
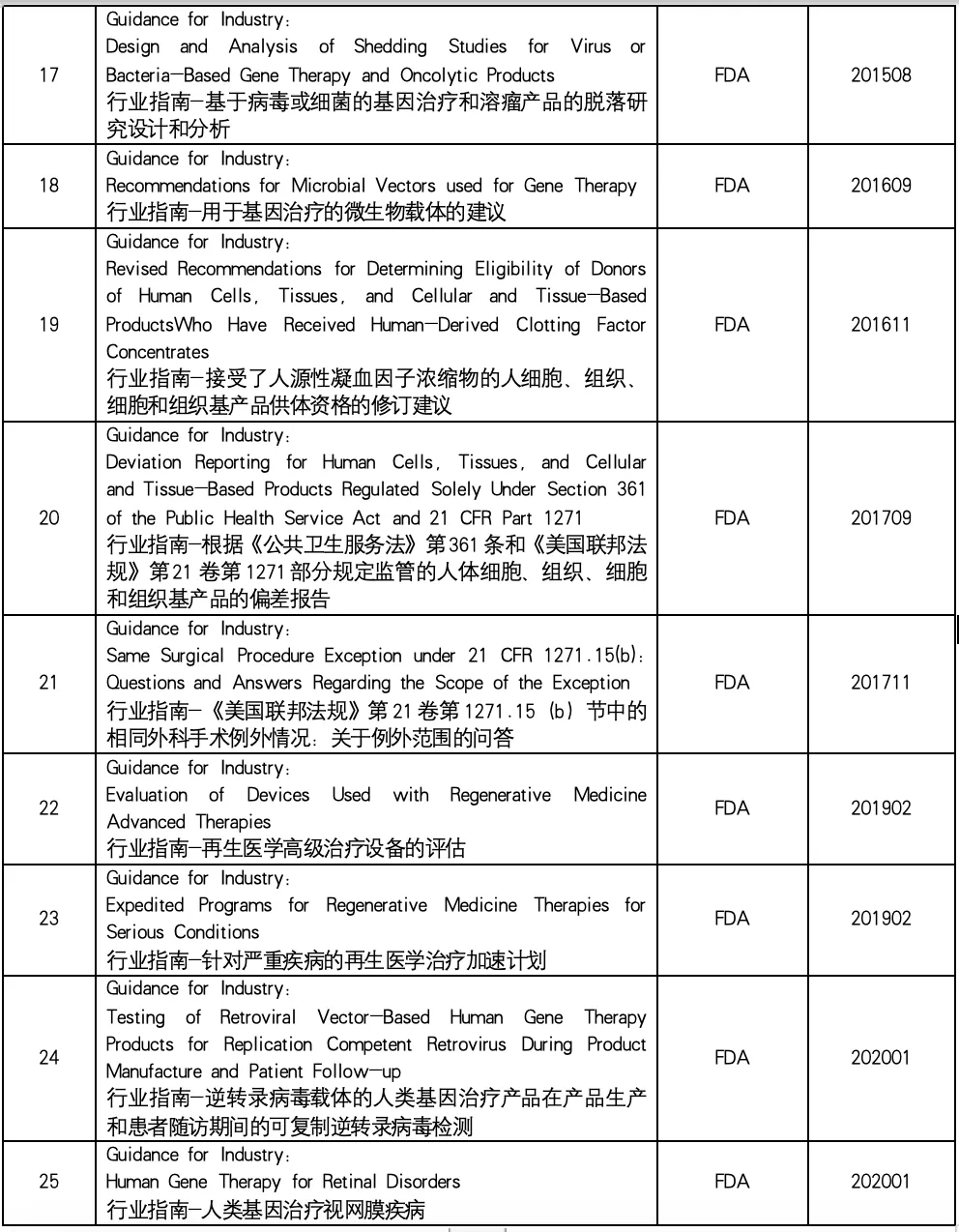
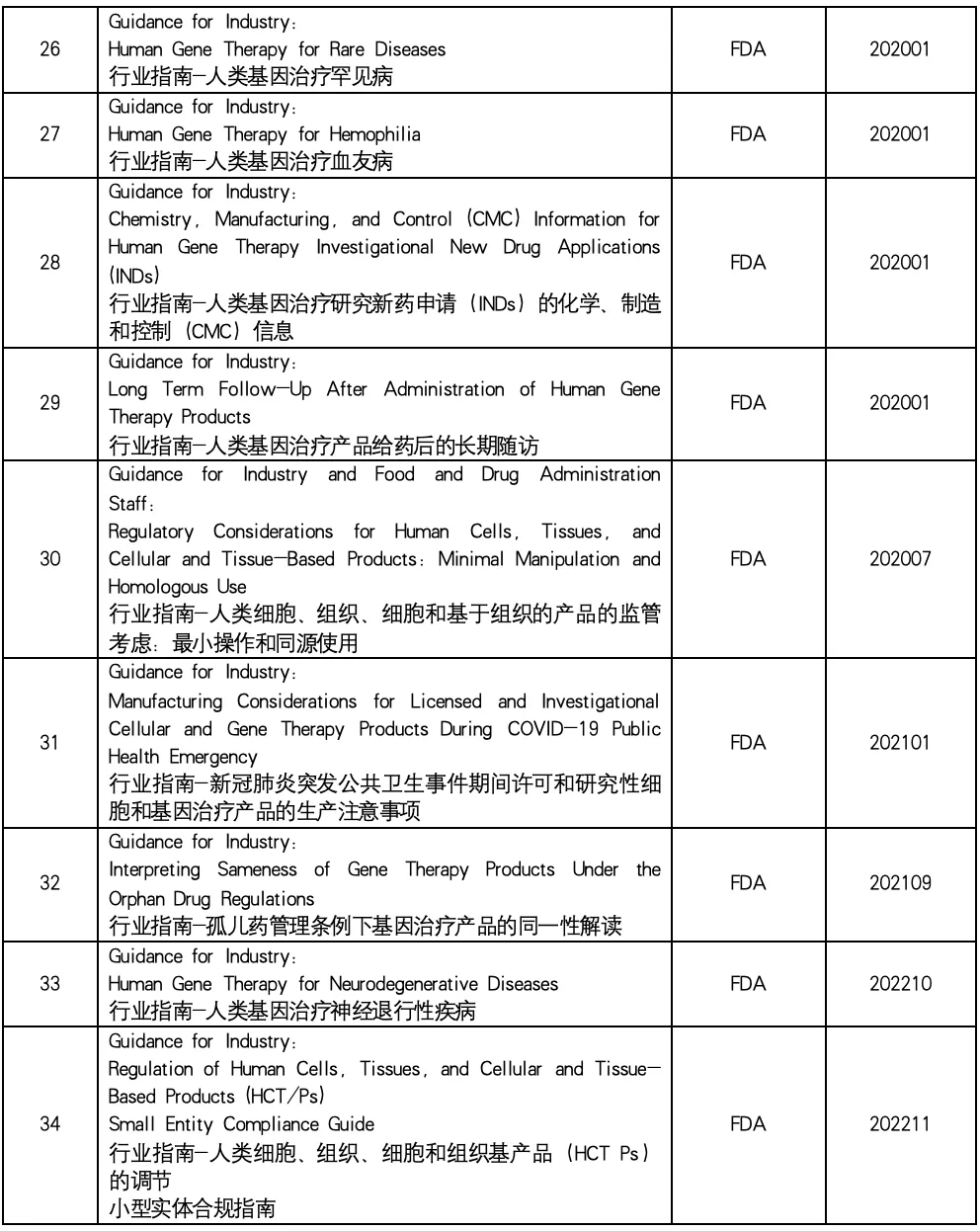
In the field of biomedicine, cell therapy and gene therapy (CGT) have become one of the most promising and dynamic research directions. These innovative therapies provide new ways to treat a variety of diseases. This paper summarizes the industry guidelines related to cell and gene therapy issued by the US Food and Drug Administration (FDA) to help readers understand the regulatory framework and regulatory trends in the field of cell therapy and gene therapy, and provide help for related research and clinical practice.