
Cell and gene therapy (CGT), as the representative of innovative medical technology, is gradually moving from laboratory to clinical application, bringing new hope to patients. China is developing rapidly in this field. On May 20, 2024, the Drug Evaluation Center (CDE) released the Annual report on the Progress of New Drug Registration Clinical Trials in China (2023). Today, we show you the highlights of the report about CGT.
1. Clinical trials of cell and gene therapy products
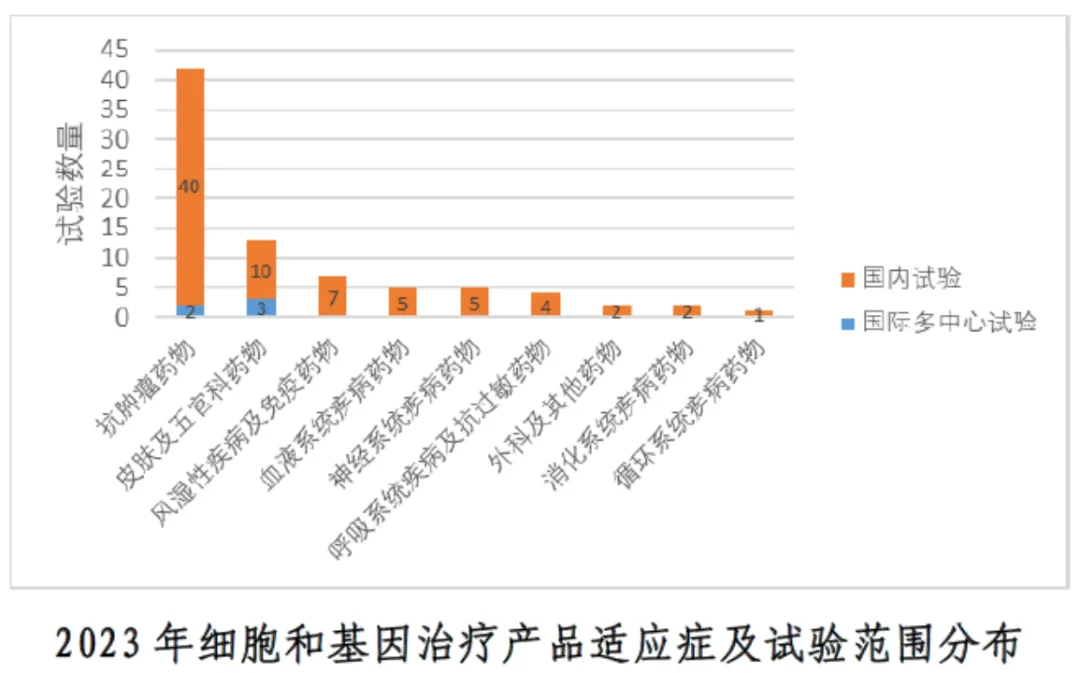
①Increase in the number of clinical trials: 81 clinical trials of cellular and gene therapy products were registered in 2023, nearly double the 46 in 2022.2 number of varieties: a total of 70 varieties were involved in 81 trials. 3 trial scope: mainly domestic clinical trials, a total of 76 (93.8%). 4 Distribution of indications: antineoplastic drugs were the main indications, with a total of 42 items (51.9%). 5 trial stages: phase I clinical trials were mainly conducted, with a total of 33 items (40.7%). The proportion of phase III clinical trials is relatively small, only 4 (4.9%).
2. Specific data from clinical trials of cell and gene therapy products
①The distribution of indications and test scope of cell and gene therapy products in 2023: the specific distribution of indications and test scope was shown, mainly in domestic trials.
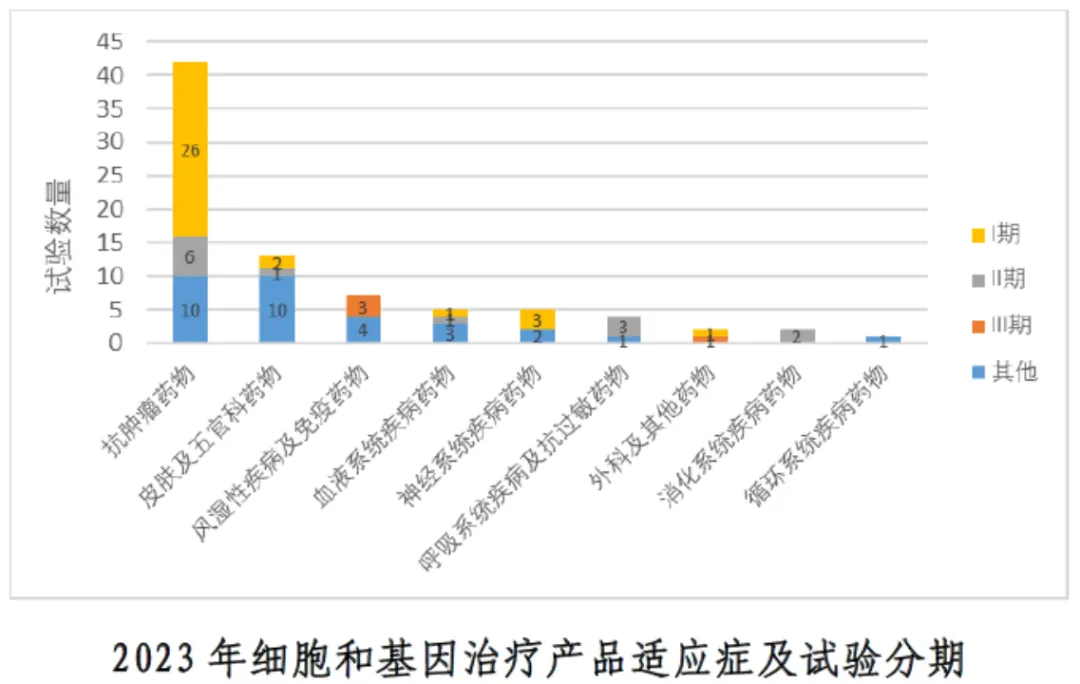
②Indications and trial stages of cell and gene therapy products in 2023: the distribution of trial stages was provided, of which phase I clinical trials were dominant.
General trend.
Growth trend: the number of clinical trials of cell and gene therapy products showed a significant increase, reflecting the increase in activity and R & D investment in this field.
Research and development focus: antineoplastic drugs, as the main indication field, show the potential and attention of cell and gene therapy in the treatment of tumors.
Clinical trials: most trials are concentrated in the early stages, especially phase I, which may indicate that many cell and gene therapy products are still in the early stages of research and development.
Conclusion.
In 2023, there was a significant increase in clinical trials in the field of cell and gene therapy in China, indicating a high level of domestic interest and investment in this cutting-edge medical technology. With the growth of the number of clinical trials and the expansion of the field of indications, it is believed that there will be more breakthroughs and progress in this field in the next few years. At the same time, the leading position of domestic clinical trials also shows China's important role in global cell and gene therapy research.
Reference: CDE- Annual report on the Progress of New Drug Registration Clinical Trials in China (2023)