
Recently, S.BIOMEDICS announced that its cell therapy TED-A9 has shown good data results in Phase 1/2 clinical trials. TED-A9 is a midbrain dopaminergic progenitor cell derived from human embryonic stem cells (hESC), which can be used in the treatment of Parkinson's disease.
One-year follow-up data from the first batch of subjects released by S.BIOMEDICS showed that three patients receiving low-dose TED-A9 showed the safety and effectiveness of TED-A9 during the one-year follow-up.
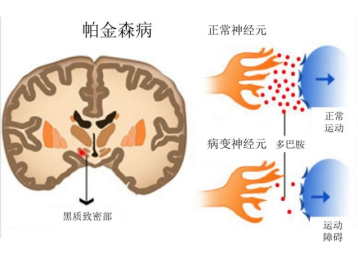
Parkinson's disease, also known as "paralysis tremors", is a degenerative disease of the central nervous system that occurs in middle-aged and elderly people. Parkinson's disease is closely related to the reduction of dopamine content in the body. It is mainly characterized by the progressive degeneration and death of dopaminergic neurons in the substantia nigra of the midbrain, which can lead to obvious movement disorders and cause various complications in the later stage of development, such as irreversible brain damage, mental decline, severe memory loss, etc. There is currently no way to cure Parkinson's disease in the world.
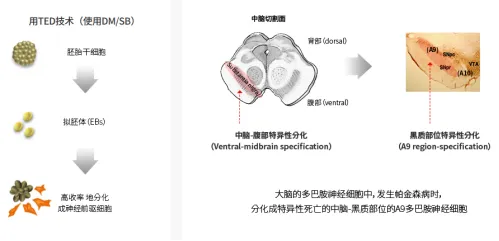
TED-A9 is a highly purified midbrain dopaminergic progenitor cell generated by treating hESC with a small molecule compound. Through surgery, TED-9 was transplanted to three segments of the putamen; the anterior, middle and posterior segments, each with three tracks, and the bilateral putamen received cell transplantation in a single operation, injecting cells at three points within each track. S.BIOMEDICS believes that by implanting these highly purified dopaminergic progenitor cells into the brain, these transplanted dopaminergic progenitor cells will mature into dopaminergic neurons, providing dopamine that Parkinson patients lack, and thus hopefully restoring patients 'motor function.

The press release further pointed out that in the safety evaluation of TED-A9, magnetic resonance imaging (MRI) and CT scan results one year later showed no relevant postoperative adverse reactions for the three patients who received low-dose (3.15 million cells) treatment.
In addition, the MDS-UPDRS Part III assessment, which objectively measures motor function, showed that the average score dropped from 61.7 points at baseline to 49.0 points after treatment one year later, an improvement of 12.7 points, indicating a significant improvement in the patient's exercise ability.
Dopamine transporter (DAT) brain imaging (FP-CIT-PET) performed a year later showed increased levels of dopamine transporter, meaning dopamine neurons may have been colonized and associated with improvement in the patient's Parkinson's symptoms.
According to relevant data, the main stem cells used to treat Parkinson's disease around the world are embryonic stem cells ESC, neural stem cells NSC, artificially induced stem cells iPSC and mesenchymal stem cells MSC, of which ESC and iPSC are currently the largest. Many companies in my country are also actively promoting stem cell-related research and application and have made significant progress.
Zehui Chenxing Biotech, which has a layout in the fields of ESC and iPSC, developed CAStem cell injection (composed of immune and matrix-regulatory cells, similar to MSCs) is differentiated from clinical grade human embryonic stem cells. The indications mainly include acute respiratory distress syndrome, acute exacerbation of interstitial lung disease, acute graft-versus-host disease, etc.
For multiple sclerosis, Emstein Bio, the first T-MSC extracellular matrix cell drug derived from human embryonic stem cells, developed stem cell drug became the world's first new drug under development that was approved by the FDA for clinical trials in 2020, and has been in the future. Preclinical research has been carried out in multiple fields, such as neuromyelitis optic spectrum diseases, adrenoleukodystrophy, osteoarthritis, inflammatory bowel disease, etc.
Shize Bio is focusing on the development of clinical grade iPS-derived cell drugs to treat nervous system diseases such as Parkinson's disease. It is the first national-level clinical study approved in my country for iPS-derived cells to treat Parkinson's disease, and the only officially approved iPS-derived cells A national-level stem cell filing clinical research project for the treatment of nervous system diseases. Shize Biotech has completed China's first clinic-grade iPS-derived cell drug transplant to treat Parkinson's disease. It is the first personalized autologous iPS-derived cell replacement transplant in China and the second in the world for the treatment of Parkinson's disease.
We look forward to the emergence of more innovative therapies in the future to promote the development of stem cell therapy and provide new ideas and methods for the treatment of other refractory diseases.
References:
1.https://pipelinereview.com/s-biomedics-dopamine-cell-therapy-for-parkinsons-disease-with-ted-a9-shows-promising-results-at-12-months-in-phase-i-iia-clinical-trial/