
Parkinson's disease not only affects the physical health and quality of life of patients, but also causes a heavy psychological and economic burden on patients and their families. To make matters worse, there is currently no effective way to cure Parkinson's disease or alter its progression. Therefore, there is an urgent need to develop new ways to treat and prevent Parkinson's disease. In recent years, with the continuous development of regenerative medicine, a large number of studies have proved that stem cells have a positive role in the treatment of Parkinson's disease, and can improve nerve function through a variety of immunological and histological measures.
It is proud that recently, Chinese researchers used a new type of stem cell therapy, namely olfactory mucosal mesenchymal stem cells (hOM-MSC) to treat 5 patients with Parkinson's disease, and achieved amazing results, the Research results were simultaneously published in the journal Military Medical Research (Military Medical Research).
Parkinson's disease
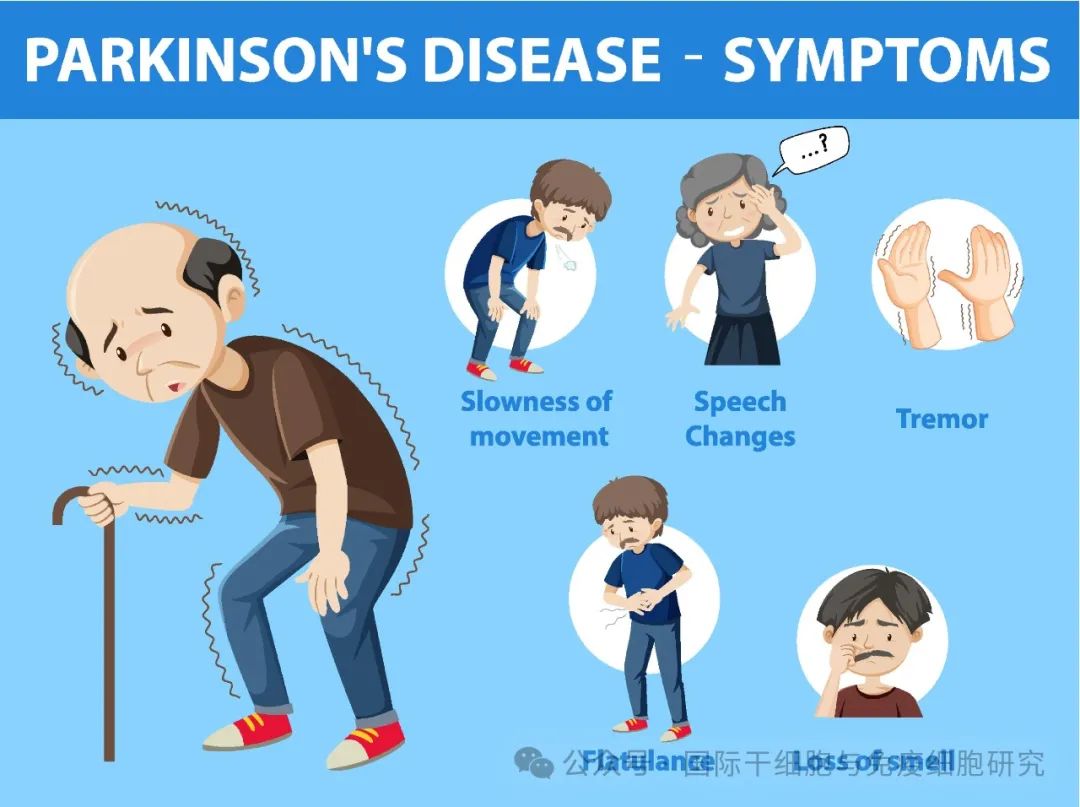
In a medical paper published in 1817, the British doctor James Parkinson described in detail a chronic, slowly progressing neurological disease characterized by tremors, stiffness, and stoop, the first description of Parkinson's disease.
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the degeneration of dopaminergic neurons in the substantia nigra (SN), in which dopamine is a chemical messenger responsible for regulating functions such as body movement.
Parkinson's disease is more common in people over the age of 60, and patients usually start with a tremor in one hand and stiffness in the body. With the passage of time, the symptoms will gradually worsen, and then appear slurred speech, gait, smell loss, dementia, depression, eating difficulties and other manifestations, seriously affecting the quality of life of patients.
Traditional treatments for Parkinson's disease and their limitations
At present, the traditional treatment of Parkinson's disease mainly includes the following three categories:
1. Medication
In the 1960s and 1970s, clinical researchers discovered that dopamine replacement therapy (such as levodopa) could be used to treat Parkinson's disease. While replacing the lost dopamine may relieve symptoms to some extent, it does not address the root cause of Parkinson's disease, which is the inability to reverse the loss of neurons. In addition, long-term oral administration of levodopa can also cause adverse reactions, and drug resistance often makes this treatment ineffective.
2. Surgical treatment
Common surgical procedures such as deep brain stimulation can partially alleviate the symptoms of Parkinson's disease, but deep brain stimulation surgery may destroy the nerve nucleus and even cause irreversible nerve damage risk.
3. Other treatments
Common include traditional Chinese medicine treatment (such as acupuncture), psychological treatment, rehabilitation treatment (such as gait training, postural balance training, language impairment training, etc.).
Why can stem cell therapy treat Parkinson's Disease?
The main pathological change of Parkinson's disease is the loss of dopamine neurons, which will be gradually lost during the occurrence and development of the disease, and this degeneration is largely irreversible.
With advances in stem cell technology, nerve repair and regeneration have become possible. Stem cells are undifferentiated primary cells that can be induced to grow into any type of cell. In recent years, a number of studies have found that stem cell therapies (such as umbilical cord mesenchymal stem cells (MSC), bone marrow MSC, fat MSC, etc.) have shown good results in the treatment of Parkinson's disease.
Stem cell therapy aims to replace the number of dopamine neurons lost in the brain by replacing dopamine-producing neurons in the substantia nigra damaged by Parkinson's disease, thereby restoring motor function and controlling the continued progression of Parkinson's disease at the source!
China has successfully completed the phase 1 clinical study of HUM-MSC in the treatment of Parkinson's disease

In 2010, researchers found that olfactory mucosa (OM) -derived mesenchymal stem cells (MSCS) isolated from the human nasal mucosa are one of the best sources of stem cells. Recently, this phase 1 clinical study of stem cell therapy for Parkinson's disease in China used such stem cells, which has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2100055021) and approved by the Ethics Committee of the Second Affiliated Hospital of Hunan Normal University (2020-390).
In this study, a total of 5 patients aged 50 to 80 years old with Parkinson's disease for more than 5 years, Hoehn and Yahr grades ≥3, and poor response to drug therapy were enrolled. Mesenchymal stem cells (HUM-Mscs) derived from the patient's own olfactory mucosa were cultured with autologous serum after admission.
1. Treatment process
The treatment process mainly includes the following steps:
① Collection of patients' nasal mucosa: First, the patients received intranasal administration of chloramphenicol during the first 3 days of collection of the olfactory mucosa; Secondly, before obtaining the tissue, the nasal cavity of the patient was first subjected to surface local anesthesia, and then a small amount of mucosal tissue (about 3 mm3 in size) was extracted from the upper lateral turbinate of the patient. Finally, the collected tissue is placed in the collection tube and stored in cold storage.
② Cultivation of olfactory mesenchymal stem cells: the collected tissues are sent to the laboratory for stem cell culture, and finally the olfactory mesenchymal stem cells (hOM-MSC) preparation is obtained.
③ Stem cell transplantation: Using the form of lumbar puncture, the prepared hOM-MSC is transplanted into the patient to complete the treatment.
2. Analysis of treatment results
After 1 to 6 months of follow-up, the neurological function of these 5 patients with Parkinson's disease was effectively improved after receiving hOM-MSCs treatment, and no serious adverse events occurred after surgery. The overall results were as follows:
① The results of Western blotting showed that TGF-β1 and CD206 protein expressions were up-regulated and IL-1β protein expression was down-regulated at 5 and 11 days after cell transplantation, and the changes were time-dependent gradient (see the following figure for details).
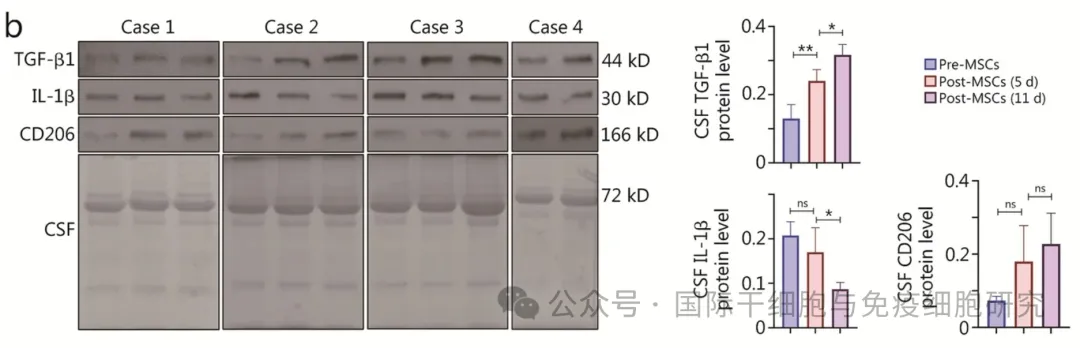
②ELISA results showed that at 5 and 11 days after transplantation, the levels of dopamine (DA) were significantly increased, the concentrations of pro-inflammatory cytokines IL-1β and TNF-α were significantly decreased, and anti-inflammatory factors IL-4, IL-10 and TGF-β1 were present after cell therapy.
3. Comparison of five patients before and after stem cell treatment
Patient 1
67-year-old woman with 20 years of Parkinson's disease. Hum-mscs were well tolerated and no serious adverse reactions were observed during the postoperative period. After treatment, the patient's mood, activities of daily living, exercise tests, and treatment complications all improved.
Patient 2
79-year-old male with a 10-year history of Parkinson's disease. The treatment of HUM-Mscs was well tolerated and no serious adverse reactions were observed. After treatment, patients showed improvement in affective, motor function assessment, and instrumental activities of daily living.
Patient 3
67-year-old male with 20 years of Parkinson's disease. Hum-mscs were well tolerated and no serious adverse reactions were observed. After treatment, the patient's mood, motor tests, activities of daily living, and treatment complications were significantly improved.
Patient 4
71-year-old male with 4-year history of Parkinson's disease. Hum-mscs were well tolerated and no serious adverse reactions were observed. After treatment, the patient's activities of daily living, motor tests, mood, and treatment complications all improved.
Patient 5
62-year-old female with 13 years of Parkinson's disease. Hum-mscs were well tolerated and no serious adverse reactions were observed. After treatment, the patient's mood, motor tests, and activities of daily living all improved (see table below).
Table 1 Comparison of five patients before and after stem cell therapy
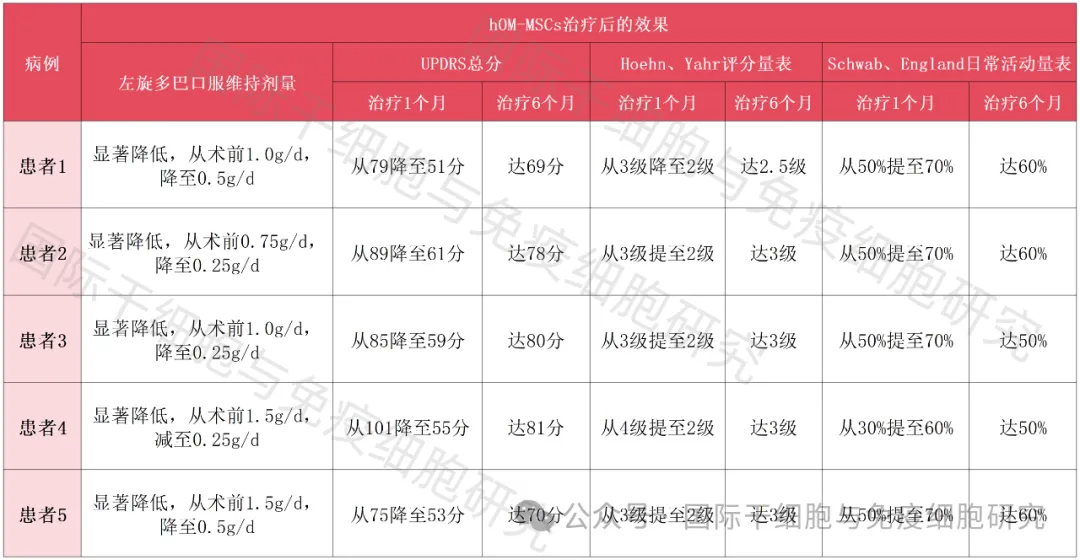
Taken together, the trial proves that Parkinson's disease is reversible in principle, and this in turn may represent an important step forward in the field of stem cell treatment for Parkinson's disease!
Small series message
Parkinson's disease is not so simple as "tremor", its severe symptoms and long course of disease will seriously affect the physical health of patients, reduce their quality of life, and the existing treatment is difficult to reverse the degeneration of dopaminergic neurons, in other words, only improve the symptoms, but can not prevent the continuous progress of the disease. The emergence of regenerative therapy represented by stem cells is expected to prevent the process of the disease from the source of the cause, bringing a ray of light to Parkinson's disease patients! Many countries in the world have also joined the research and development battlefield of stem cell treatment of Parkinson's disease, such as Bayer in Germany and the research team of Concorde Hospital in China.
The results of this "Phase 1 clinical study on the treatment of Parkinson's Disease with hOM-MSC" carried out by Hunan scholars are encouraging and have left a strong mark in the treatment of Parkinson's disease! I hope that with the efforts of researchers in various countries, Parkinson's disease will eventually be conquered one day!