In Japan, research and development of stem cell therapies as part of regenerative medicine has received great attention and active promotion from the government. The Japanese government clearly recognizes that compared with traditional chemically synthetic drugs or biological products, regenerative medical products have unique treatment principles and efficacy due to their inherent biological activity and self-renewal capabilities, representing a new medical model that is expected to provide innovative treatment options for many refractory diseases.
In today's medical community, stem cell therapy has become a high-profile cutting-edge field. The Japanese government not only prioritizes the research and development of stem cell therapies in its national strategy, but also provides strong support at the regulatory and policy levels to ensure that this innovative technology can protect the rights and interests of patients while playing its role in treating various refractory diseases. Huge potential in diseases.
In order to ensure the safety and effectiveness of regenerative medical products, Japan has established a strict legal and regulatory system that comprehensively regulates the research and development, production and application processes of regenerative medical products. What is particularly noteworthy is that the Japanese government has included some regenerative medical products that have undergone strict review and received restricted approval into the reimbursement scope of national health insurance. This shows that once these products are officially included in the National Medical Insurance Drug List or the National Medical Insurance Medical Materials List, patients can enjoy reimbursement from national health insurance, thereby reducing the financial burden and improving treatment accessibility. Currently, Japan's insurance system already covers some innovative cell transplant therapies.
Summary of Japanese Stem Cell Application List
Japan's Ministry of Health, Labor and Welfare has approved a series of mesenchymal stem cell (MSC) treatment projects. These projects target a wide range of indications, including but not limited to diabetes and its complications, such as diabetes foot, stroke, arthropathy, chronic pain, spinal cord injury, arteriosclerosis, erectile dysfunction, premature ovarian failure, and menopausal syndrome. They are also involved in the field of beauty and anti-aging.
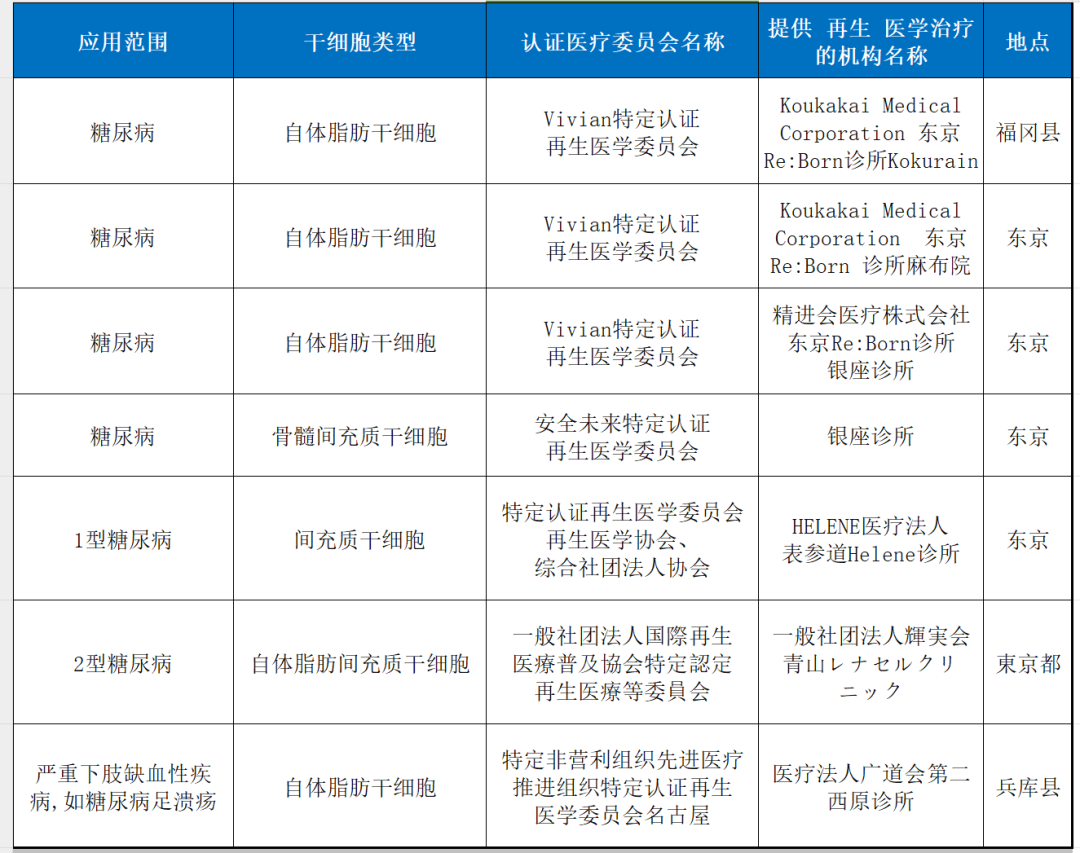
1. Diabetes and diabetic foot
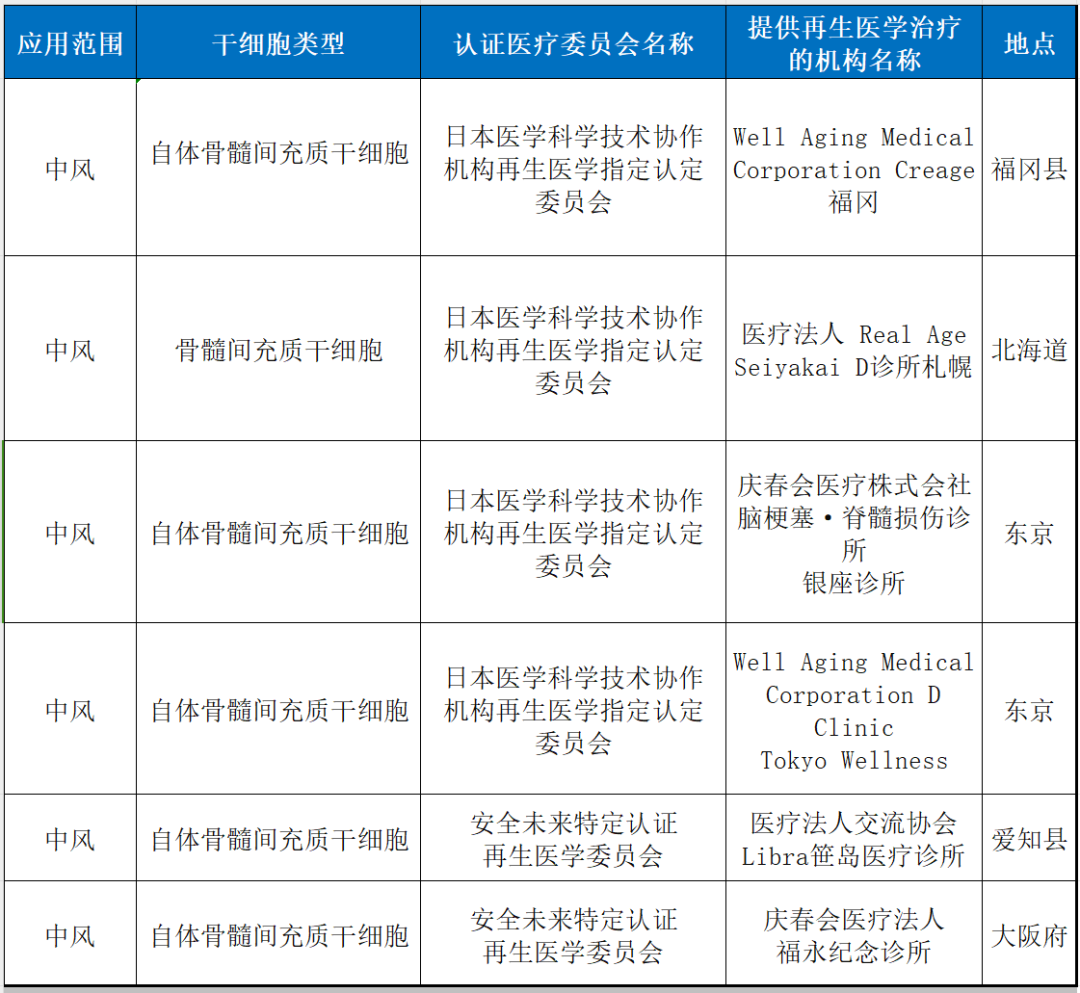
2. stroke
3. joint disease
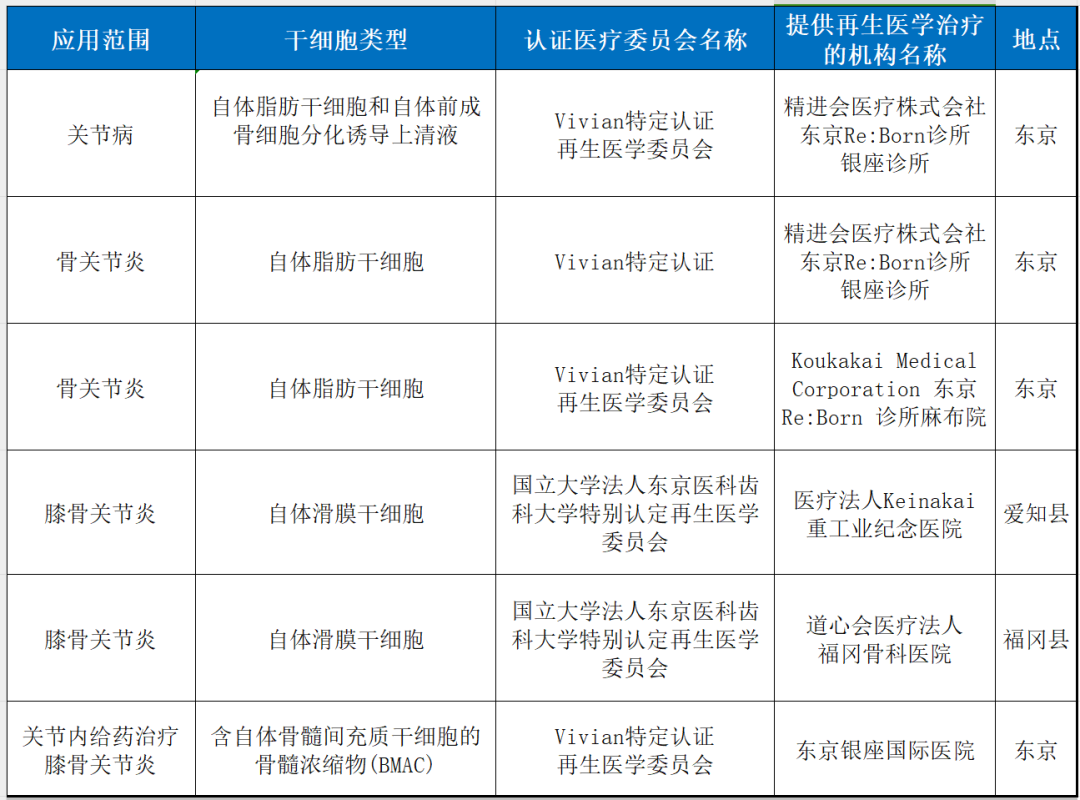
4. chronic pain
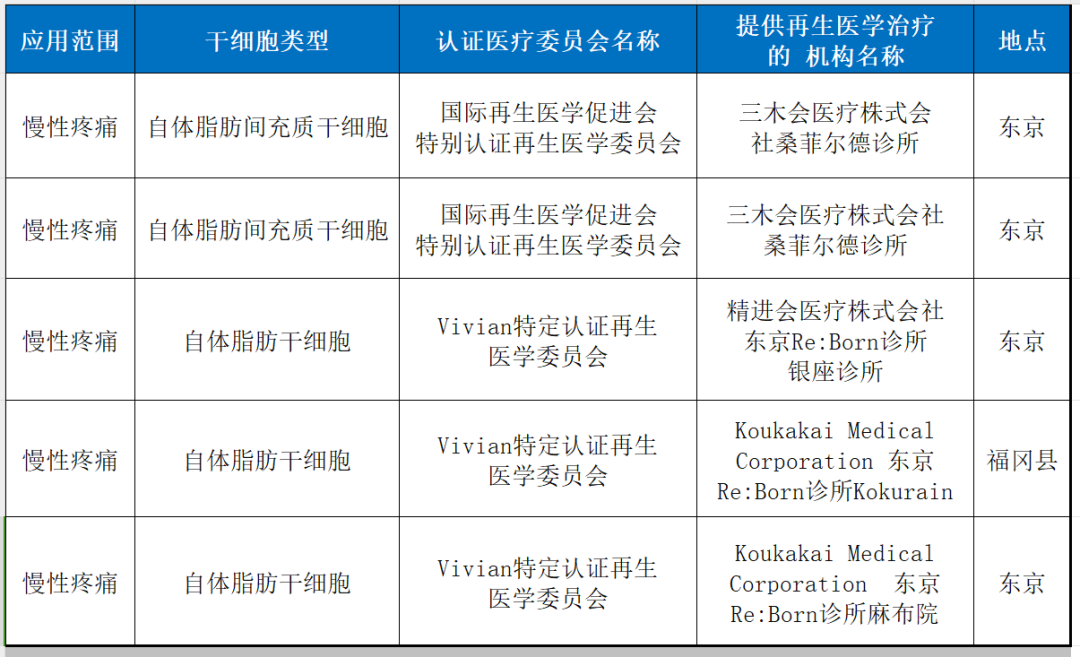
5. spinal cord injury
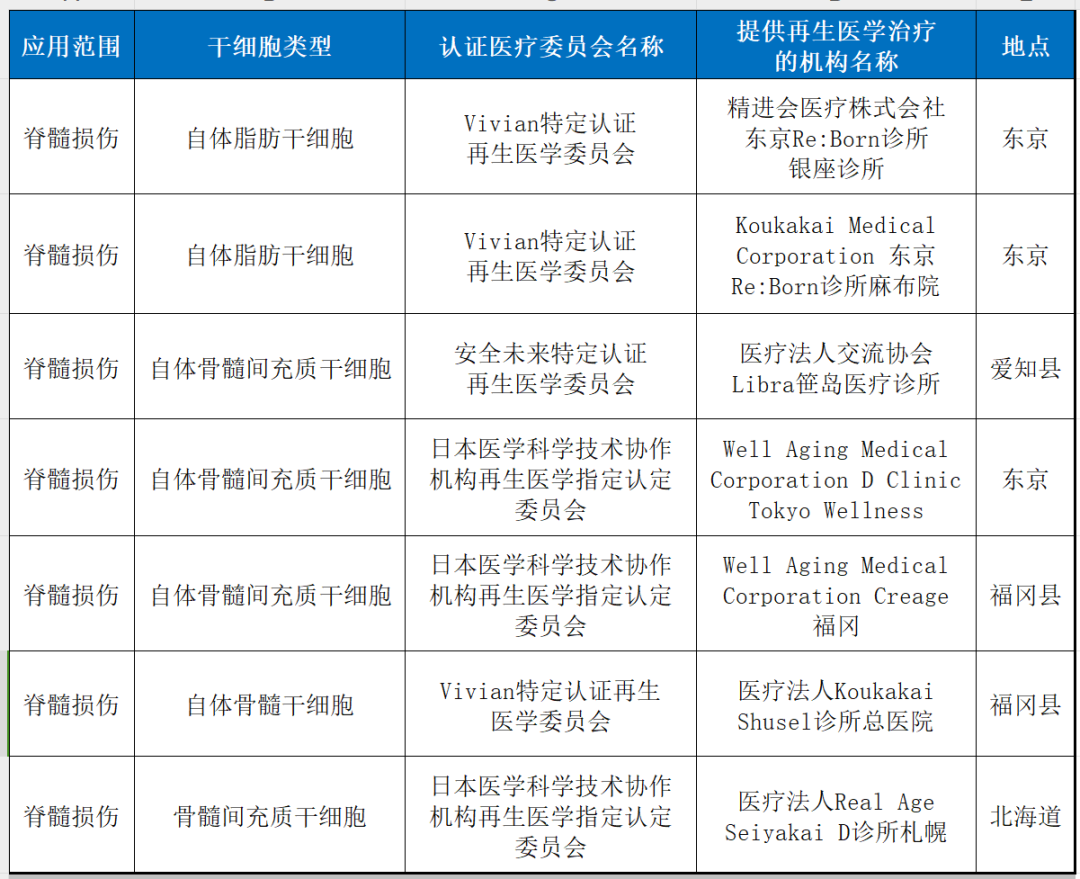
6. other neurological system diseas
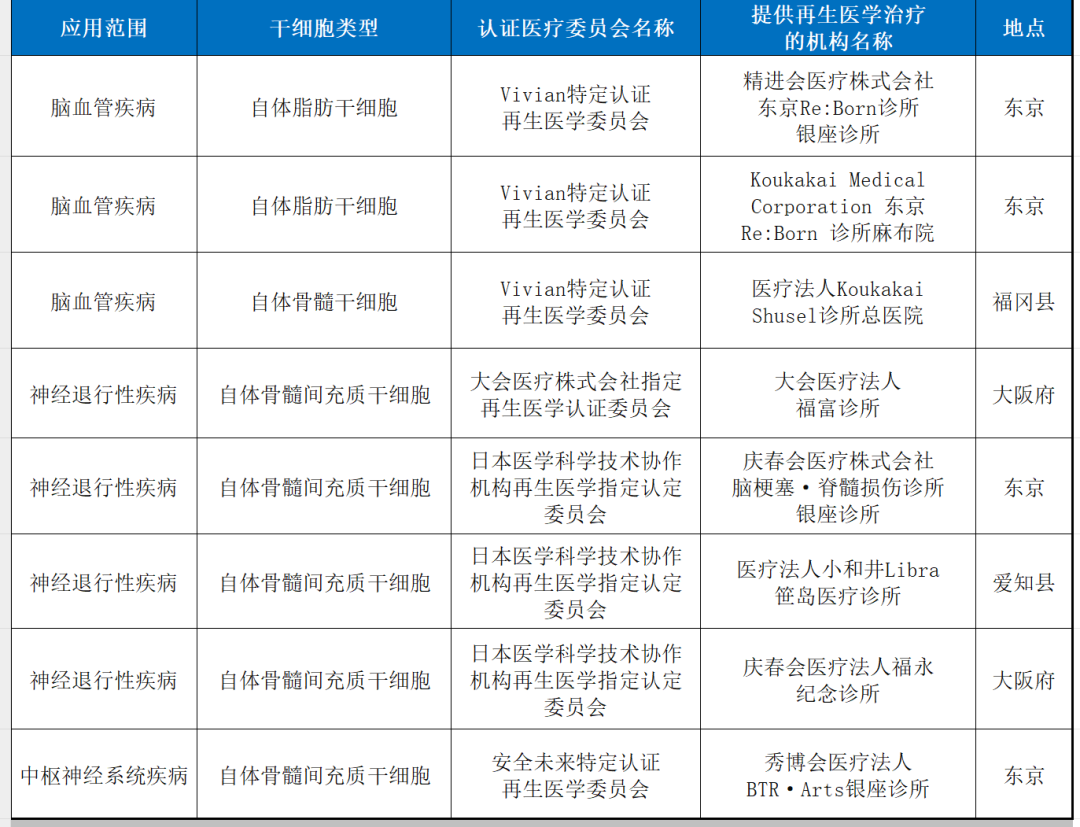
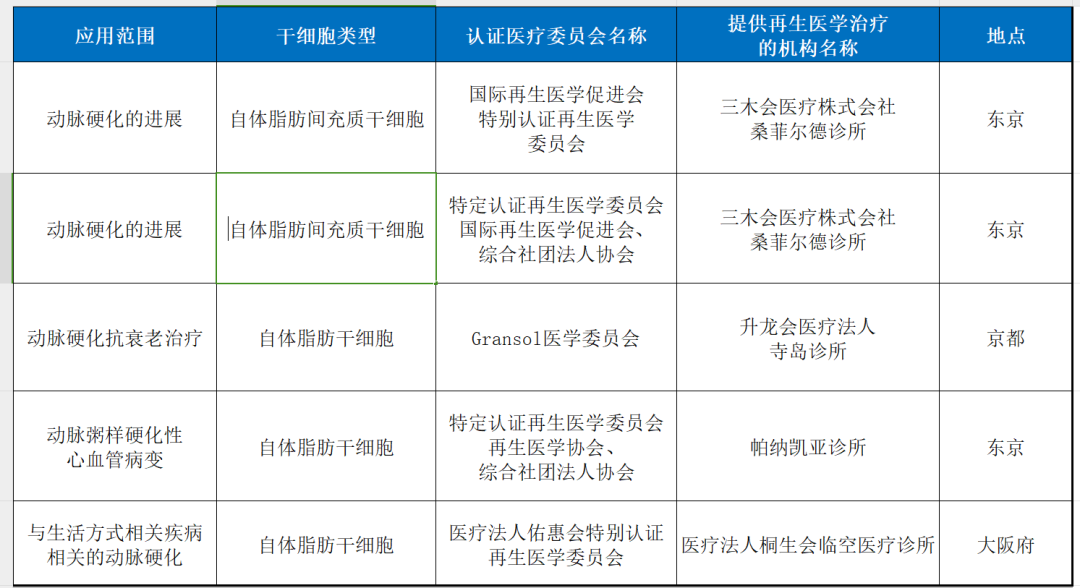
7. arteriosclerosis
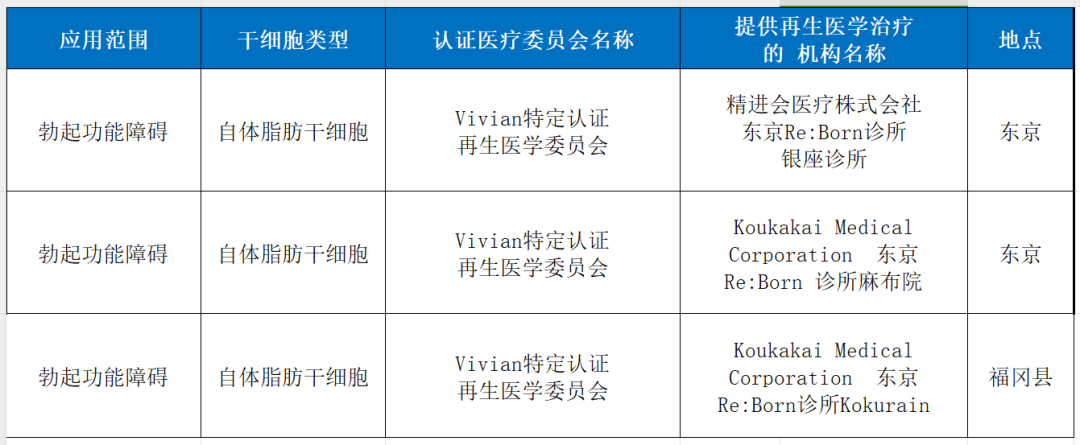
8. Erectile dysfunction
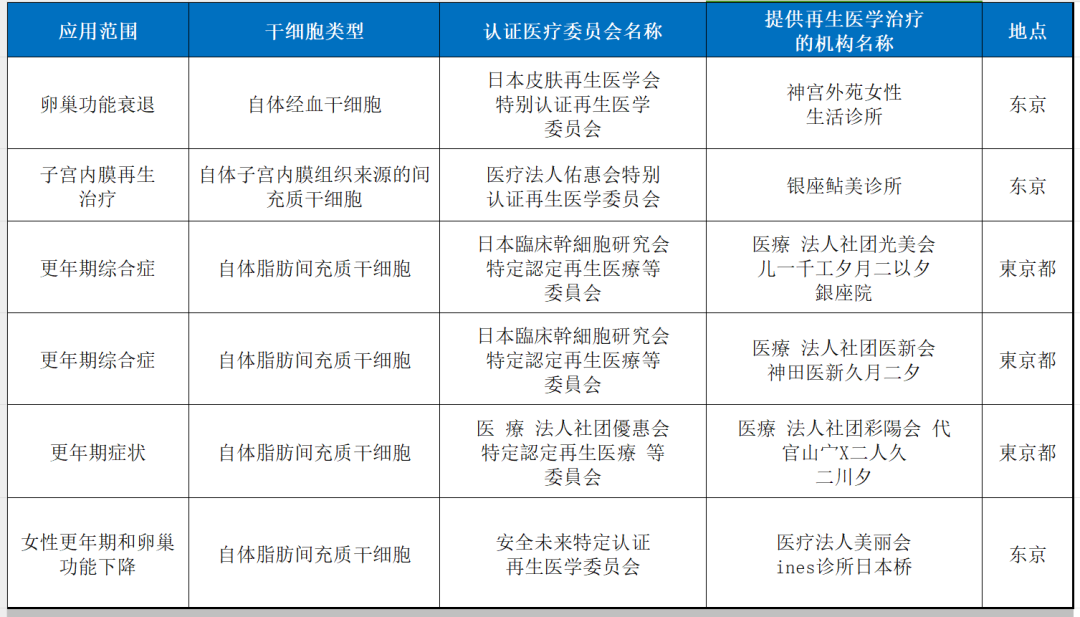
9. Premature ovarian failure and menopausal syndrome
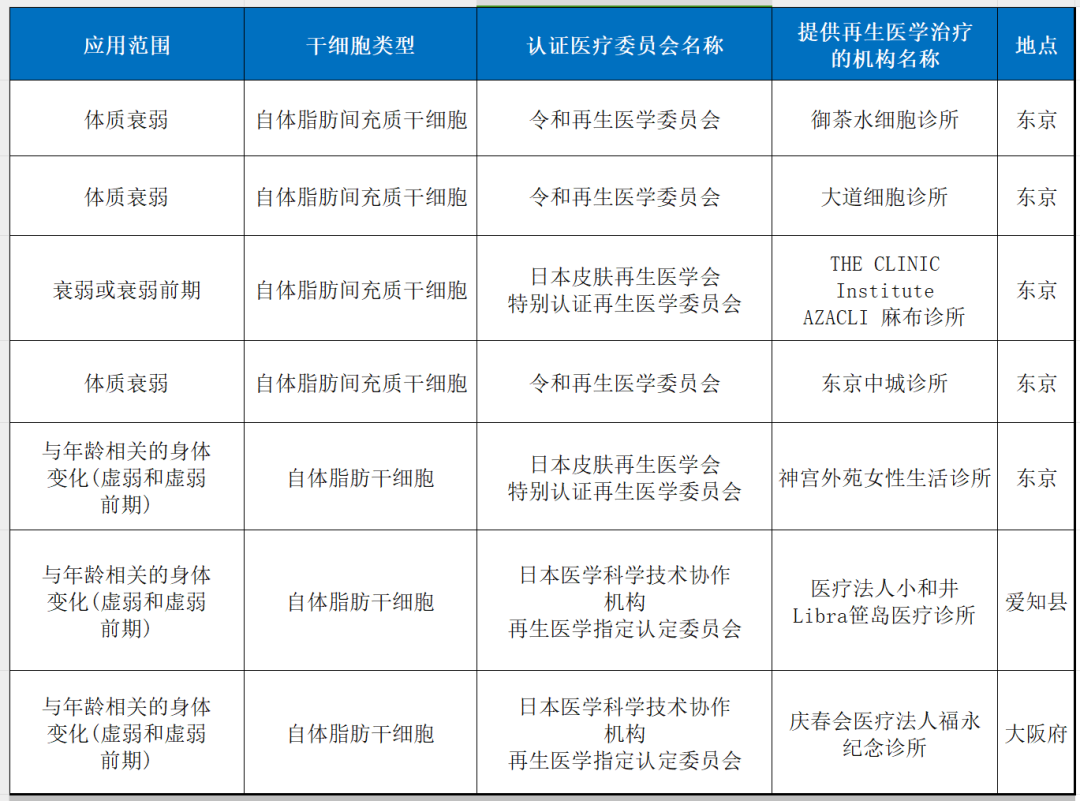
10. Sub-health status
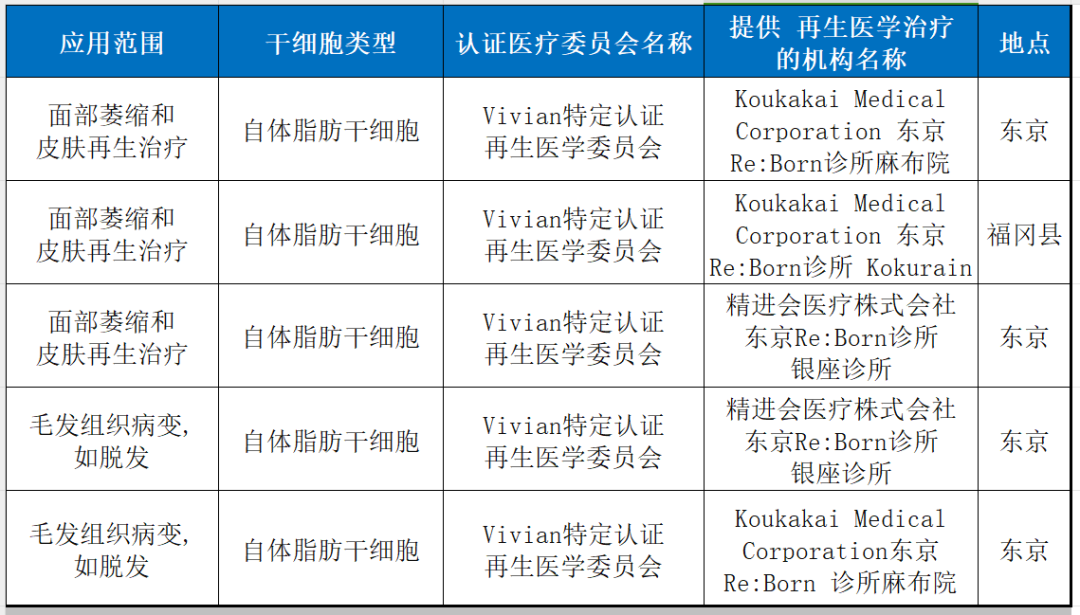
11. Beauty and anti-aging