
In recent years, with the continuous development of regenerative medicine in my country, a number of new stem cell drugs have been approved, involving the prevention and treatment of many chronic diseases such as stroke, diabetic foot, chronic obstructive pulmonary disease, ulcerative colitis, and chronic heart failure. Patients suffering from chronic diseases bring new hope!
Regulatory policies and new drug approval process for stem cell therapy
Countries have slightly different regulatory policies on stem cells. Take my country as an example. Since the issuance of the "Notice on Improving the Supervision and Management of Stem Cell Clinical Research in 2019" in 2019, my country has adopted a "quasi-dual-track management model", that is, the development and research of stem cell therapy are jointly supervised by the "State Food and Drug Administration" and the "Health Commission"! The approval process for new stem cell drugs mainly includes the following steps:
1. New Drug Clinical Research Application (IND)
When a stem cell product or new clinical drug successfully passes preclinical trials, it is necessary to submit a new drug clinical research application to the U.S. Food and Drug Administration (FDA) or the State Food and Drug Administration (China). If the FDA does not reject the application within 30 days of submitting the application, the clinical research application for the product will be deemed valid and relevant clinical trials can be conducted.
2. New Drug Application (NDA)
After completing all Phase 3 clinical trials of this product, the research data and data can be compiled and summarized, and then submitted to the U.S. FDA (or our country's Food and Drug Administration) to ensure the effectiveness, safety, and quality controllability of the marketed drug. sex.
The FDA usually completes the NDA approval of the product within the specified time frame of 6 months. But usually, due to too many application materials, the approval time for many new drugs may be longer.
As of the first half of 2024, a full summary of new stem cell INDs in my country
As of the first half of 2024, more than 60 new stem cell INDs have been approved by the Center for Drug Evaluation (NMPA) of the State Food and Drug Administration of China, covering a variety of chronic and refractory diseases.
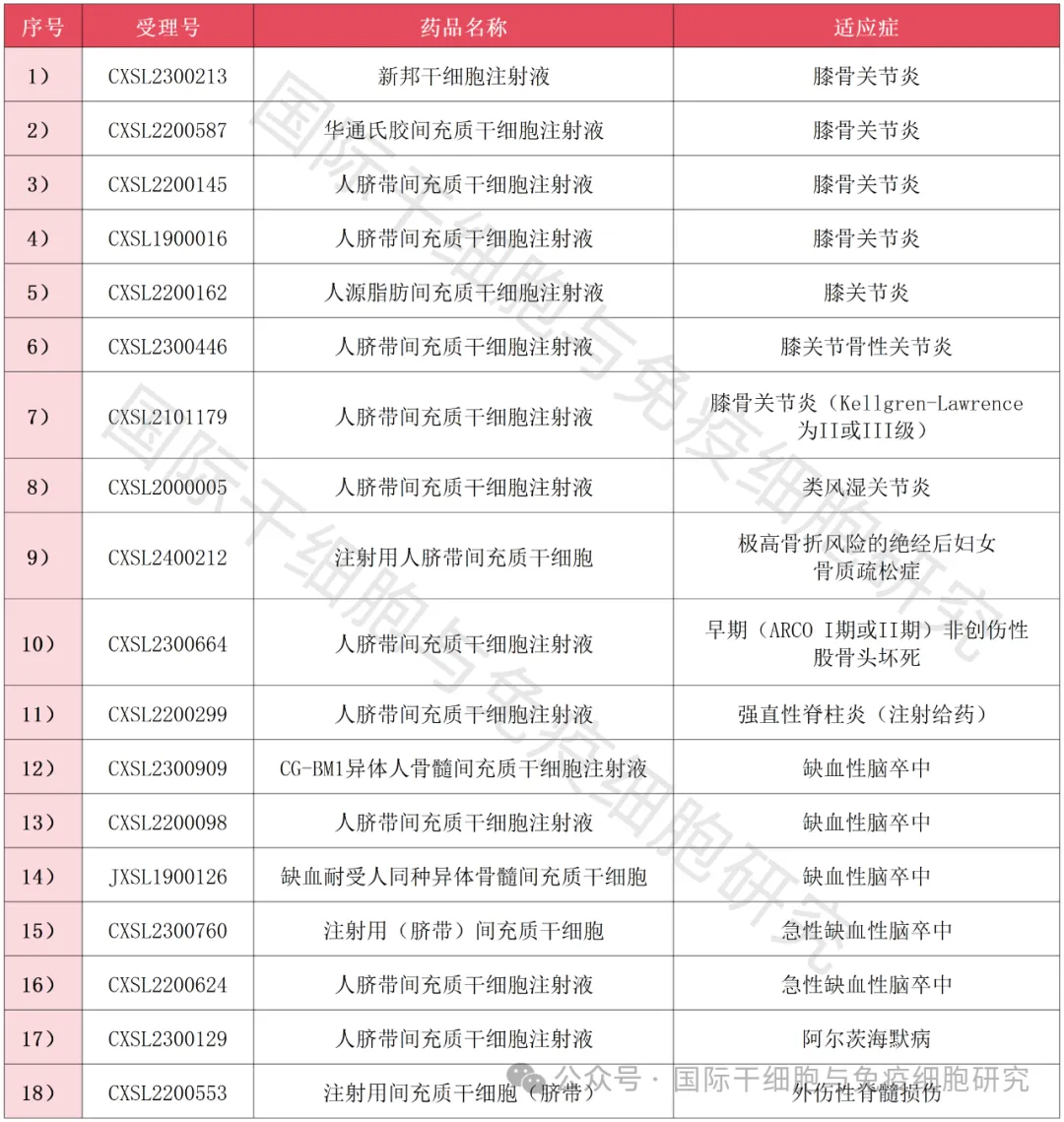
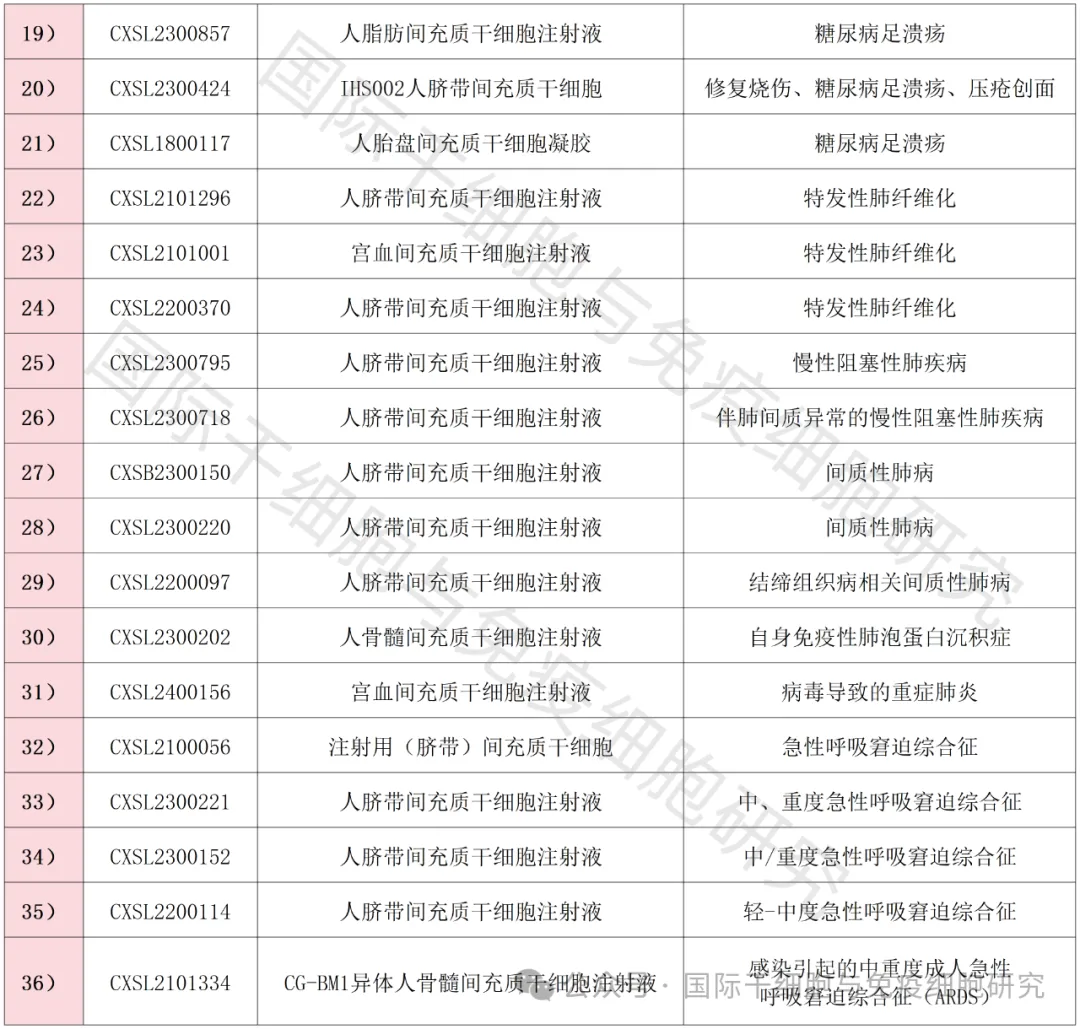
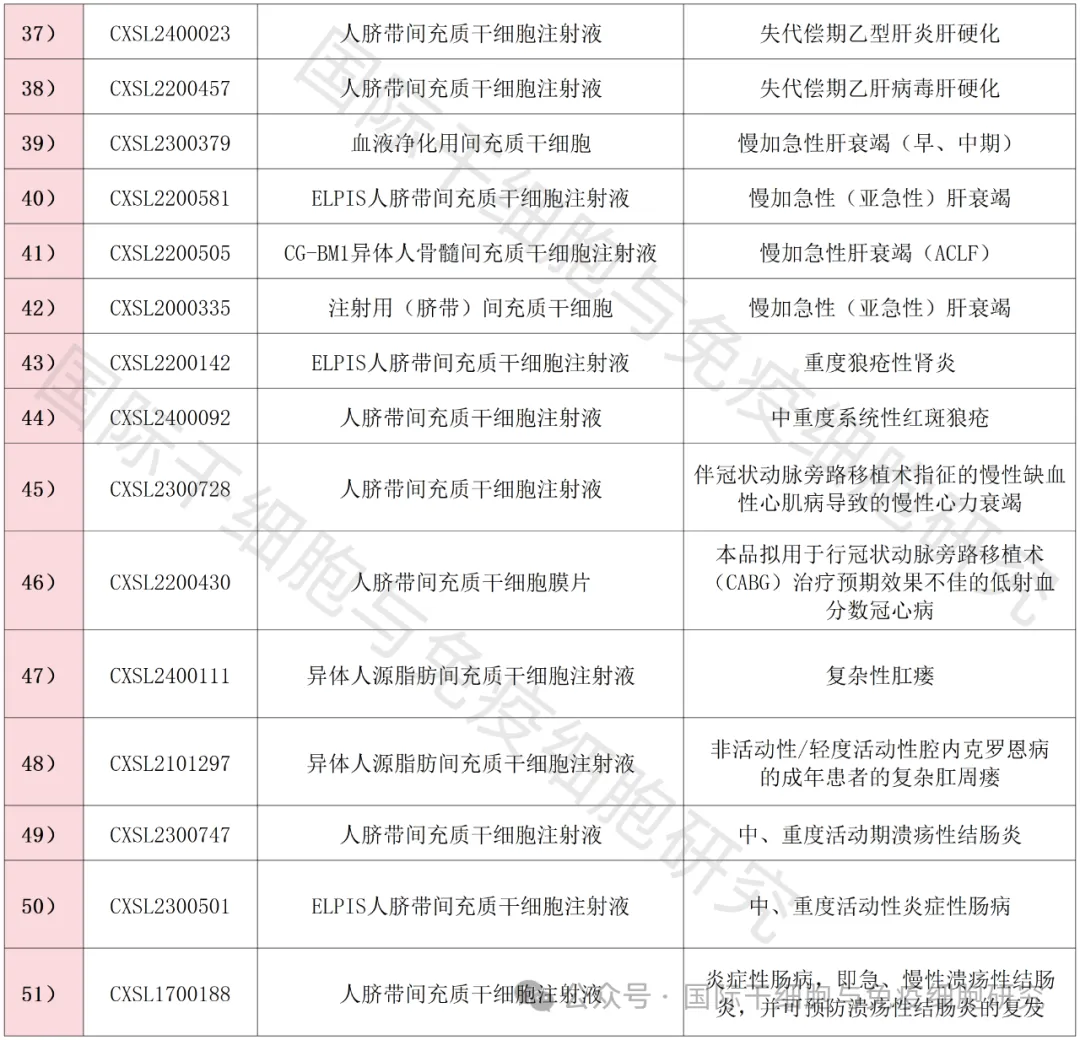
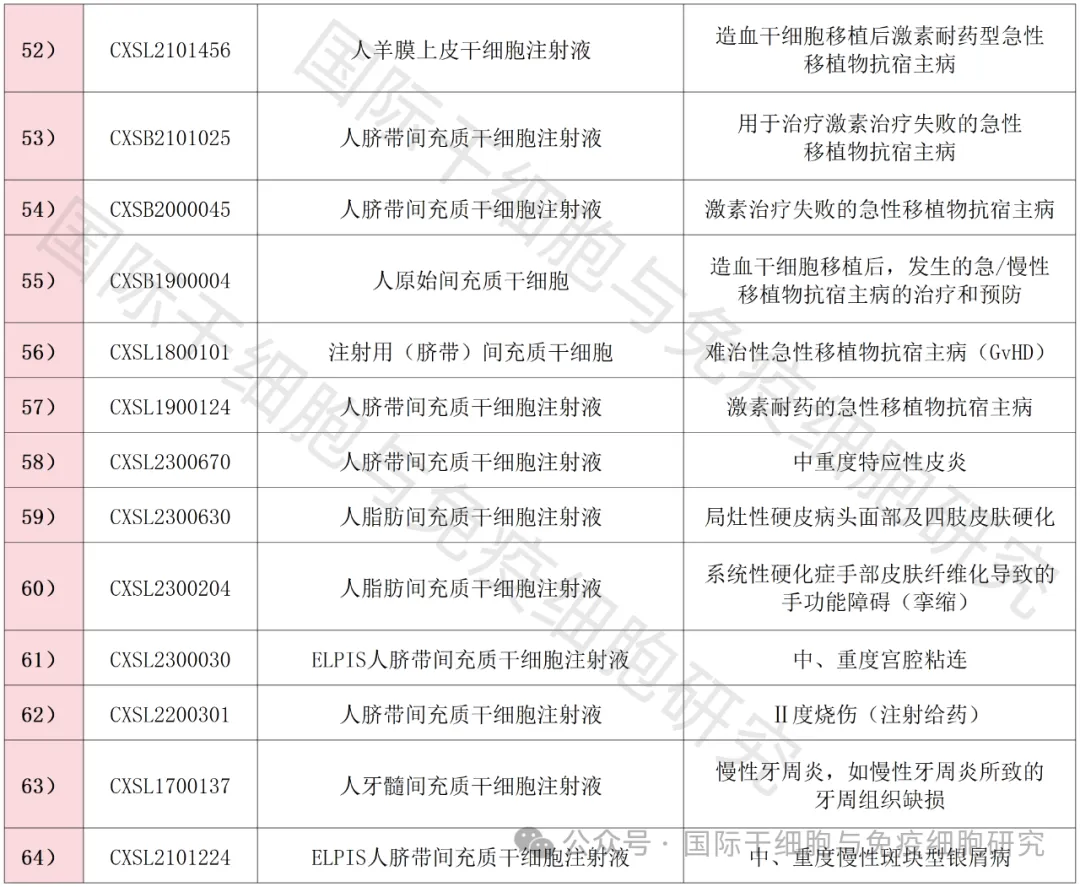
▲ Data comes from "NMPA"
Mmessages
With the development of regenerative medicine, researchers are constantly seeking breakthroughs from the cellular level, striving to grasp the laws of cell life and activity, thereby gradually realizing the beautiful vision of overcoming diseases and delaying aging! In recent years, my country's stem cell research and development field has also followed the footsteps of countries such as Japan and the United States. New drug INDs have increased year by year, and the diseases covered are becoming increasingly extensive!
Although there are currently no approved commercial stem cells in my country, the good news is that domestic patients can now choose to go abroad for medical treatment, or seek stem cell help through health management companies, specific medical service institutions, or participating in clinical trials.
Resources:
[1]Maldonado,et al.Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy.J Biol Eng 17,44(2023).
https://jbioleng.biomedcentral.com/articles/10.1186/s13036-023-00361-9#citeas
[2]https://www.cde.org.cn/