

In 2007, Nobu Yamanaka of Japan and Yu Junying of China announced at the same time that they successfully transformed human somatic cells into induced pluripotent stem cells (iPSC) and caused a sensation in the world. The characteristics of iPSC are similar to those of embryonic stem cells (ESC)-infinite expansion + tridermal cell differentiation potential. iPSC is derived from human somatic cells and has unlimited access to materials, making it an ideal source of "seeds" for cell treatment of various diseases.
In addition to cell therapy, iPSC also has great application value in disease modeling, drug development and discovery, personalized medicine, toxicology testing, etc.
01 iPSC reprogramming method
At present, iPSC reprogramming technology is developing rapidly, and reprogramming factors can be introduced into somatic cells through a variety of technologies, which are mainly divided into two categories: integrated reprogramming technology and non-integrated reprogramming technology.
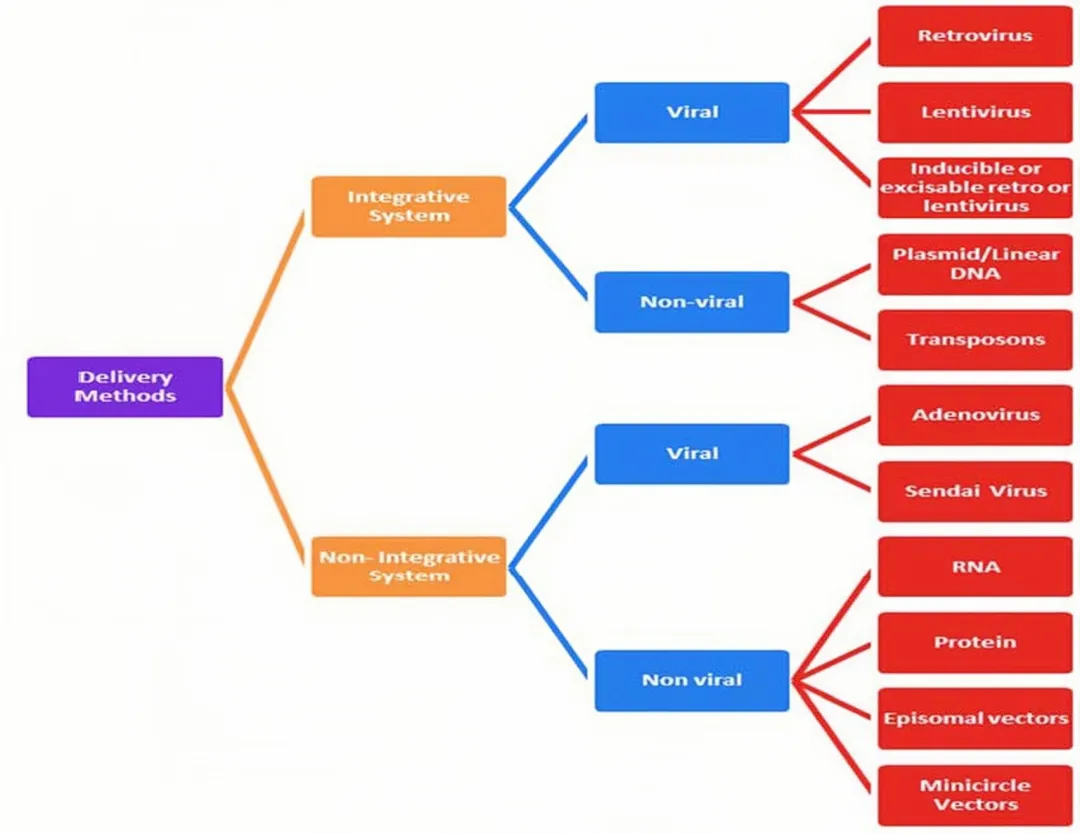
Faced with many iPSC reprogramming methods, which one is more appropriate?
Dr. Yu Junying, one of the international pioneers of iPSC technology, once said: The most important thing to choose for iPSC cell therapy is to see which method can obtain high-quality iPSC and ensure its drug-friendliness. In fact, all roads lead to Rome, and obtaining high-quality iPSC is the most important thing. From the perspective of drug research and development, clinical applications should consider the balance between benefits and risks. When choosing an iPSC reprogramming method, you need to evaluate the advantages and disadvantages of each method, taking into account the safety and quality stability of reprogrammed cells.
Each type of reprogramming technology has its own advantages and disadvantages. For example, reprogramming technology of viral vectors can achieve efficient reprogramming, but the residual virus risks being reactivated; non-virus-mediated integrated reprogramming methods are safer, but iPSC reprogramming efficiency is relatively low; for chemical small molecule reprogramming, the operation is simple and highly controllable, but it is necessary to focus on whether the selected small molecule will affect the stability of the cell's genome and cause genetic mutations.
02 iPSC Preclinical Trial
2006-2007 iPSC reprogramming was first achieved on mouse and human somatic cells in 2000. It was subsequently found that rat and non-human primate somatic cells could be reprogrammed using the same transcription factors, indicating that the mechanism of inducing cellular pluripotency is consistent in mammals. In addition, iPSC has been induced from multiple species such as dogs, rabbits, horses, pigs, cattle, and sheep.
03 iPSC Applications
Application of iPSC in disease modeling
iPSC has the potential to unlimited self-regeneration and directed differentiation into any specific cell in the body. iPSC can be induced from somatic cells from anyone, regardless of genetic composition. By obtaining somatic cells from patients with complex refractory diseases, establishing disease models is expected to provide new ways for disease mechanism research and drug development.
Case 1: A study used a non-invasive method to isolate renal proximal tubular epithelial cells (RPTEC) from the urine of patients with multiple sclerosis (MS), reprogram them into iPSC and induce them to differentiate into primary neurons step by step. Analysis showed that iPSC-neurons in MS patients were normal in morphology and function, and mature neuron markers were tested positive. This study established a practical and reliable MS model that will help further study the mechanism of MS and discover new therapeutic targets.
Application of iPSC in drug screening
Many drugs have shown good results in animal experiments, but fail to show efficacy or experience unforeseen adverse reactions in humans, making them unable to be marketed. Costs can be greatly reduced if toxicity to humans can be predicted in the early stages of drug development, but the difficulty lies in the limited and unstable nature of human samples. The emergence of iPSC technology is expected to solve this problem. At present, iPSC technology has made significant progress in pharmacological and toxicological screening. It can generate cells from patients with specific diseases and improve the accuracy of efficacy and toxicity verification of drug treatment trials. Generating different iPSC-cell lines for specific diseases helps examine genetic and potential epigenetic differences in different populations; helps test the personalized therapeutic effects of drugs at the individual level and supports personalized treatment concepts based on patient genetic or molecular profiles; can greatly reduce the number of animals used in drug trials, detect human toxicity early in preclinical trials, and reduce the risks and costs associated with clinical trials.
Although iPSC technology helps in drug screening, iPSC residues and undifferentiated precursor cells may exist during the targeted differentiation of iPSC. Therefore, when using iPSC-derived cells, it is crucial to ensure the purity of the target cells.
Case 1: The study used iPSC-derived neurons from Alzheimer's disease (AD) patients for drug screening to screen for compounds that reduce the accumulation of abnormally phosphorylated Tau protein (pTau). Among the more than 1600 compounds tested, 42 were found to be effective in reducing pTau levels, including known pTau modulators and newly discovered compounds. Due to the known role of cholesterol metabolism in AD, the focus of research has been on Cholesterol targeting compounds, especially statins. Cholesteryl ester (CE) accumulation has been observed in the brains of AD patients and transgenic mouse models, and studies have shown that lowering CE levels by various compounds that act through different mechanisms can reduce pTau levels at multiple phosphorylation sites in iPSC-neurons in familial Alzheimer's disease (FAD), sporadic Alzheimer's disease (SAD) and non-dementia control (NDC) subjects.
It is worth noting that CE has different effects on amyloid (Ab) and pTau levels, and CE independently regulates pTau and Ab, suggesting a more complex relationship between these landmark proteins and challenging the idea of a single linear relationship linking Ab to Tau in disease progression.
Application of iPSC-derived cell therapy in disease treatment
iPSC has many advantages such as unlimited expansion, multi-directional differentiation potential, and easy gene editing. The potential of being derived into various adult cells allows it to be applied to multiple disease fields and is expected to solve the clinical needs of many complex and refractory diseases.
Case 1: Heart disease
The heart has limited self-repair ability and often leads to heart failure after injury. Differentiation of iPSC into cardiomyocytes is a potential future therapy.
A study efficiently induced and differentiated human iPSC into cardiac precursor cells and implanted them into a rat model of myocardial infarction. The results showed that compared with the untreated group, rats in the treated group had some retention of cardiac function at week 10. Histological analysis demonstrated the presence of transplanted cells in cardiac tissue, which suggests cell transplantation and its potential feasibility in protecting cardiac function after injury.
Case 2: Diabetes
iPSC-derived cell therapy can provide a potential new treatment for diabetes and diabetic wounds.
A study for the first time compared iPSC-derived insulin-secreting cells (IPCs) from individuals with type 1 diabetes, type 2 diabetes and non-diabetic. It was found that iPSC-IPCs from patients with type 1 diabetes and type 2 diabetes expressed similar markers such as Pdx-1, MafA, Beta2/NeuroD and insulin secretion levels as those from non-diabetic patients. The study reveals the potential of using patient-specific iPSC for autologous transplantation treatment of type 1 and type 2 diabetes.
Another study explored the healing ability of iPSC-endothelial cells (EC) in mouse wounds. Studies have found that iPSC-EC treatment accelerates wound healing and increases blood vessel formation during the initial healing phase. In the first week after treatment, endothelial cell markers increased significantly and wound perfusion was enhanced. On the 14th day of treatment, wound collagen content increased, indicating that wound healing was faster. However, most cells were lost within 48 hours after treatment, and iPSC-EC was tracked down within two weeks, suggesting that the wound healing effect may be mainly due to its secretion of factors. Further research is needed in the future on how to extend the survival time of iPSC-EC in vivo and expand the therapeutic effect.
04 Progress in iPSC clinical trials
iPSC-derived cell therapies have therapeutic potential for a variety of refractory diseases and have attracted many companies around the world to deploy in this field. A number of cell therapy products based on iPSC technology have entered clinical trials, with indications involving tumors, knee osteoarthritis, Parkinson's disease, heart failure, graft-versus-host disease, etc.
Foreign companies that have entered the clinical stage include: Cynata, Fate, Heartseed, Century, and Aspen
Domestic companies include: Zhongsheng Traceability, Huo De Bio, Ruijian Pharmaceutical, Qihan Bio, Chengnuo Medicine, Aierpu Regenerative Medicine
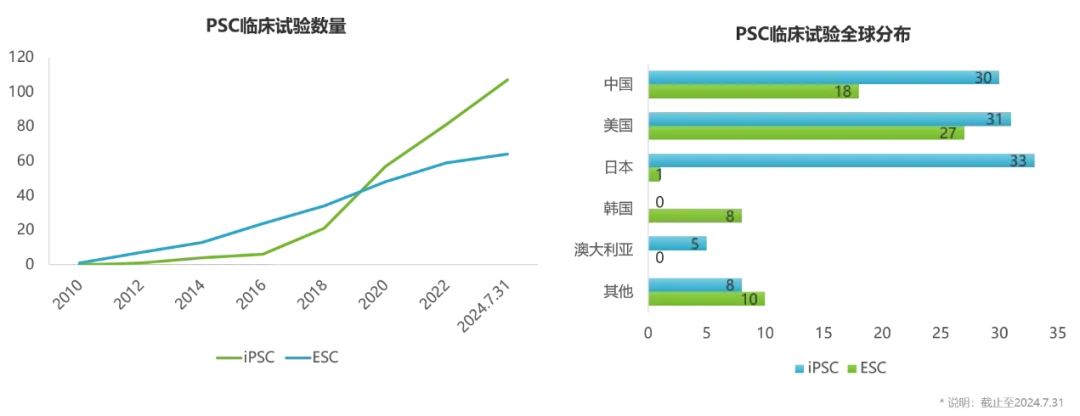
As of July 31, 2024, more than 100 iPSC clinical trials have been registered on ClinicalTrials, and the total number has exceeded ESC clinical trials.
05 iPSC risks and challenges
iPSC has huge potential in the biomedicine field, but it still faces many challenges. The development process of iPSC-derived cell therapies is very complex, including multiple links such as reprogramming, library building, production, establishment of quality standards, and storage. Each link requires strict quality control.
In addition, safety issues such as tumorigenicity and immunogenicity have been receiving widespread attention. Let's take a look at what industry celebrities say about tumorigenic risks and immune matching issues.
Dr. Yu Junying, one of the international pioneers of iPSC technology, once said: Not only iPSC, tumorigenicity is a problem that all cell therapies have to face, especially after long-term in vitro culture of cells.
However, considering the source of tumorigenicity of iPSC technology cells, it can be controlled in several aspects. On the one hand, the residue of pluripotent stem cells will cause tumorigenic risks; at the same time, during the cell differentiation process, there may be cases where there may be more precursor cells remaining. Like neural stem cells, they have a strong amplification ability. If excessive amplification, it may also lead to the formation of tumors; on the other hand, genetic mutations generated during cell culture are also one of the causes of tumorigenic disease.
These can use highly sensitive detection methods to detect pluripotent stem cell residues in cell products, target cell purity, cell genome stability, and in vivo tumorigenesis tests to minimize risks. For example, iPSC cell products need to be transplanted into immunodeficient animals for a long time to observe whether they have the possibility of forming tumors, which can greatly reduce the risk of forming tumors. The tumorigenic risks of different cell products are different, and for each product, its risks need to be carefully analyzed.
As far as immunomaturing is concerned, we can prepare super donor iPSC, or we can prepare universal pluripotent stem cells through gene editing.
So far, pluripotent stem cells, especially iPSC, are the only technical platform that can solve the problems of cell quality, cell number and immune matching. Therefore, in terms of drug quality, iPSC-derived cell therapy products have very strong drug properties.
06 Summary
iPSC technology has broad application prospects in the fields of cell therapy, disease modeling, drug screening, etc., but it also faces many challenges. With the continuous maturity of iPSC technology and further clinical verification in the future, it is expected to provide new hope for cure for many currently without effective treatments.
References:
Int J Surg. 2024 Jul 4. doi: 10.1097/JS9.0000000000001892IF: 12.5 Q1 .