
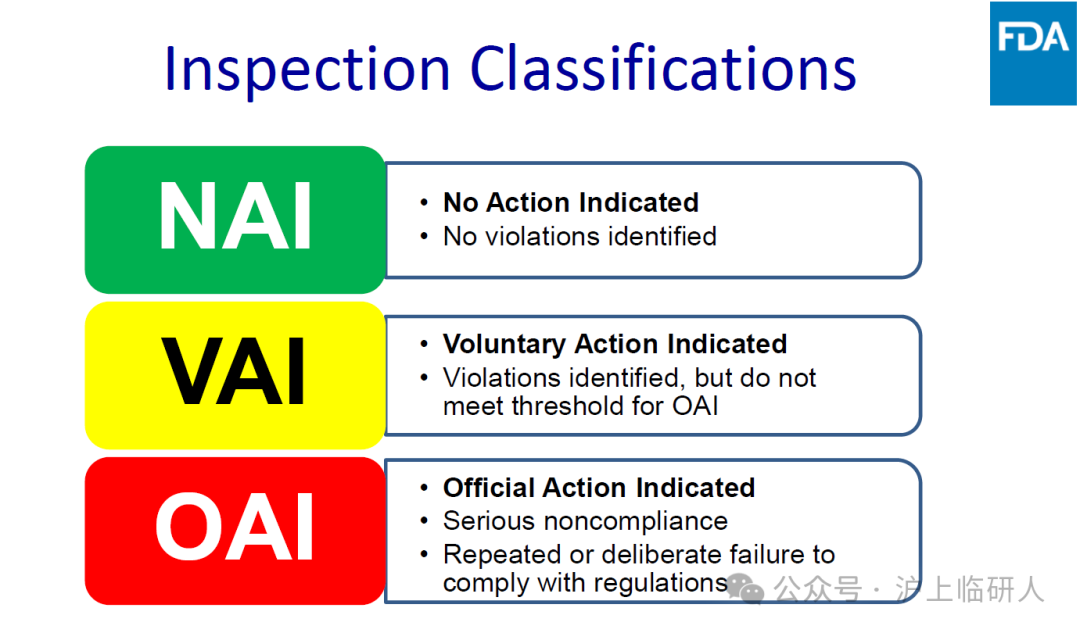
The conclusions of on-site verification of clinical trials in the United States are divided into:
NAI: "No administrative action required"
VAI: "Identify problems and voluntarily take administrative measures"
OAI: "Serious problems require official administrative measures"
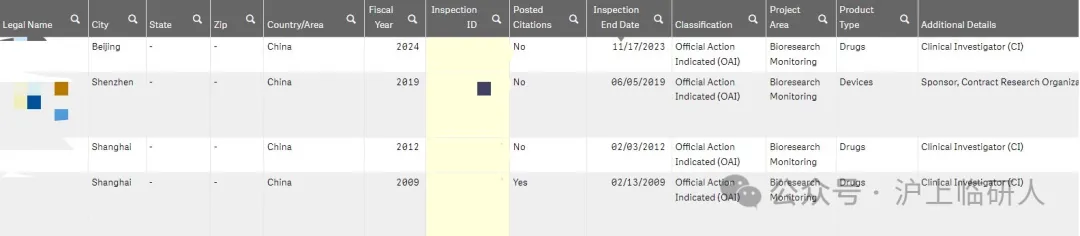
From 2009 to August 2024, after my country accepted FDA inspections, it was concluded that "serious problems require official administrative measures" were four times. The occurred in 2009, 2012, 2019 and 2024 respectively. The first two times were before "722", and the 2019 inspection was aimed at the sponsor. Therefore, this time in 2024 was the first time that "OAI" appeared in Chinese clinical trial institutions after the "722" incident.
Professor Li Ning of the National Cancer Center elaborated on three points during last year's clinical research ward round:
The quality of clinical trials in China has reached the world's leading level;
China's clinical research quality supervision system has reached the world's leading level;
The speed and evaluation system of clinical research in China have reached the world's leading level.
Professor Li Ning compiled and compared the results of FDA on-site inspections at clinical research institutions in Japan, China, South Korea, France, the United Kingdom and the United States from 2016 to 2023, as shown below. The research quality of Chinese institutions exceeds that of developed countries such as South Korea, France, the United Kingdom and the United States, and is second only to Japan. Moreover, during the on-site verification of Chinese clinical trial institutions, the FDA did not find any "serious problems requiring official administrative measures."
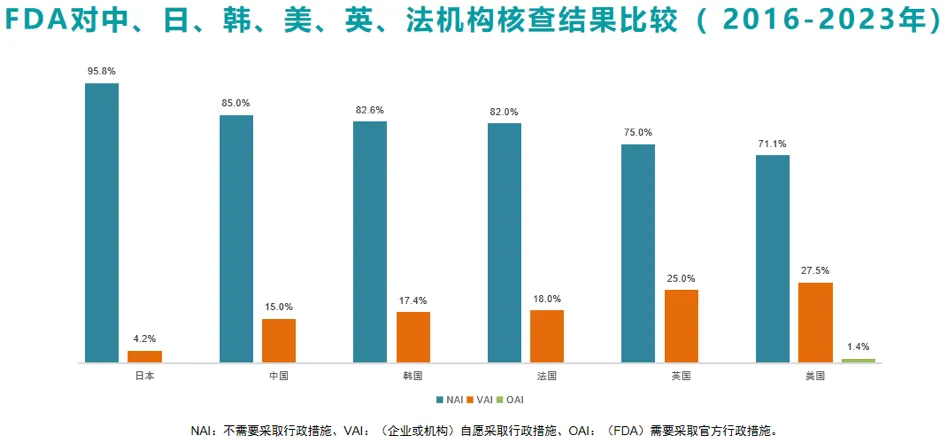
Question: Is the quality of clinical trials in China really up to standard?
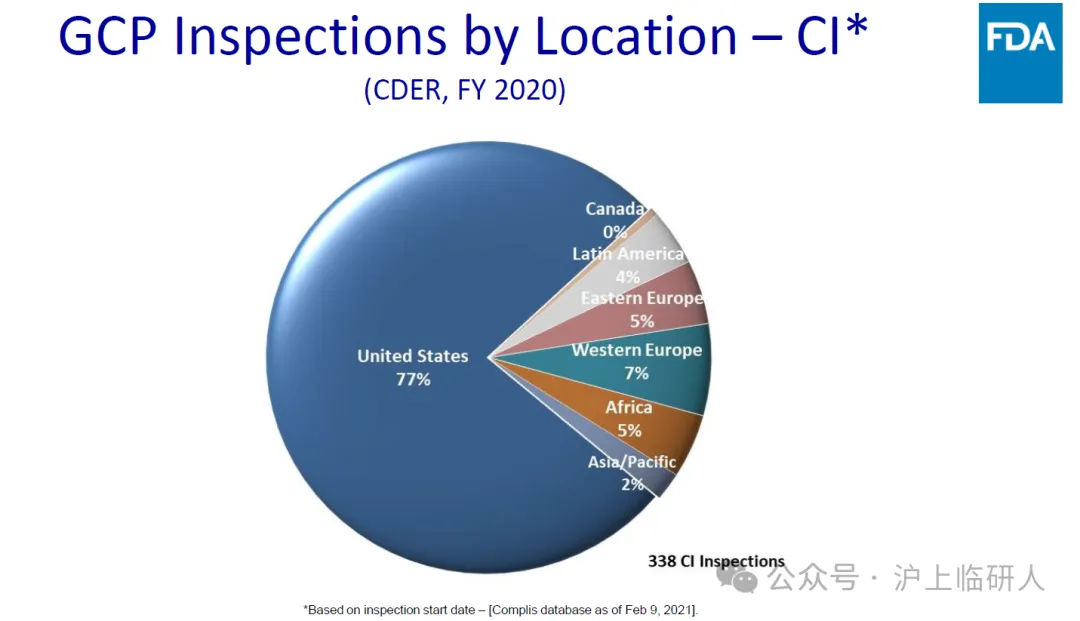
We don't think so. The number of FDA clinical trial on-site inspections in the United States is more than ten times that of any other overseas place. Since 2009, my country's clinical trial institutions have only received FDA inspections a total of 62 times, which is comparable to Japan and South Korea. The FDA's clinical trial on-site inspections in Britain and France are about twice that in my country, so that everyone can better understand why the FDA did not find "OAI" in on-site inspections of the above-listed overseas clinical trial institutions before 2023. Everyone in clinical trials is very sensitive to the sample size, which is obviously relatively low.
This "OAI" is the first time that the FDA has conducted on-site inspections of Chinese clinical trial institutions after the "722" incident. After the "722" incident in 2015, with the joint efforts of Chinese supervision and all industries, the quality of Chinese clinical trials has indeed achieved rapid progress. But we cannot be blindly optimistic. The current quality of clinical trials in my country is not optimistic, and efforts are still needed!