
"Black Myth: Wukong" is like a stone thrown into a calm lake, arousing magnificent ripples. This visual feast not only revived the childhood memories of countless players, but also built an unprecedented game experience framework with the classic element of "Seventy-Two Changes" as the core.
In a science and technology program, CCTV Department 1 invited academician of the Chinese Academy of Sciences and director of the Institute of Zoology of the Chinese Academy of Sciences to popularize knowledge of stem cells. Academician Zhou Qi said: Stem cells are like "Sun Wukong" and can become countless selves. They can also become other cells and are the source of all cells.
There are more than 200 cells in the human body, including nerve cells that transmit information, blood cells that carry oxygen, and skin cells that play a protective role. There is also a type of cell that is the source of all these cells, which is stem cells.
Some cells in our bodies have the same abilities as Sun Wukong. They can not only use the "avatar technique" to replicate themselves, but also "transform" and become other cells. This kind of "Sun Wukong cell" is a "stem cell". The "stem" in "stem cells" is not the "stem" of "dry", but the "stem" of "trunk", which means that they can constantly change in different directions during proliferation, like branch branches, and finally form various cells.
In contrast to "stem cells", most cells in our body, such as nerve cells, muscle cells, epithelial cells of the skin, and villi cells of the small intestine, do not have the ability to "transform." They can only "honestly" do their "job" and cannot "meddle in their own business", that is, change themselves to do the things of other cells. These cells are called "differentiated cells" or "somatic cells". There are more than 200 different types of somatic cells in our body, which "perform their duties" and "work together" to complete various physiological activities in the body. If this is the case, why do we still need "stem cells"?
This is because although people can live for decades or more, the life span of many cells in the human body is very short. For example, the life span of skin epithelial cells is only more than 20 days, white blood cells can live for a few to ten days, and villi cells in the small intestine can only live for two or three days. These cells need to be replaced in time. This is the task of "stem cells" in the body. "Stem cells" are cells that do not "differentiate" and can proliferate and differentiate as needed to become cells that need to be replaced. Therefore, stem cells are the "reserve" of human cells.
Who are the real stem cells that can change in 72?
However, there are many types of stem cells in the human body, such as totipotent stem cells, pluripotent stem cells and all-purpose stem cells. Which one is the stem cell that will truly transform into "72"?
With the development of fertilized eggs in humans, embryonic stem cells differentiate into various tissues and organs and are no longer pluripotent. Therefore, if you want to get pluripotent stem cells that can truly "72" transformation, you must destroy what could have become a new life. The embryo of life is destroyed, and this obviously has ethical issues.
There is a type of stem cell that has similar differentiation potential to embryonic stem cells, but there is no ethical controversy over the use of embryonic stem cells. They are induced pluripotent stem cells (iPS cells/iPSCs).
iPS cells are known as the "fighting saint Buddha" among stem cells. iPS cells usually choose to collect human epidermis, peripheral blood or kidney cells for induced reprogramming. Because the source is convenient to collect and because it comes from its own cells, there is no problem of immune rejection.

iPS cells are known as the "fairy water" in the cell world, a pluripotent stem cell that can be permanently preserved, infinitely expanded, and can be "transformed into 72".
iPS cells can differentiate into almost all cells in the human body, such as liver cells, ovarian cells, and also include cells that are difficult to regenerate, such as cardiomyocytes and nerve cells.
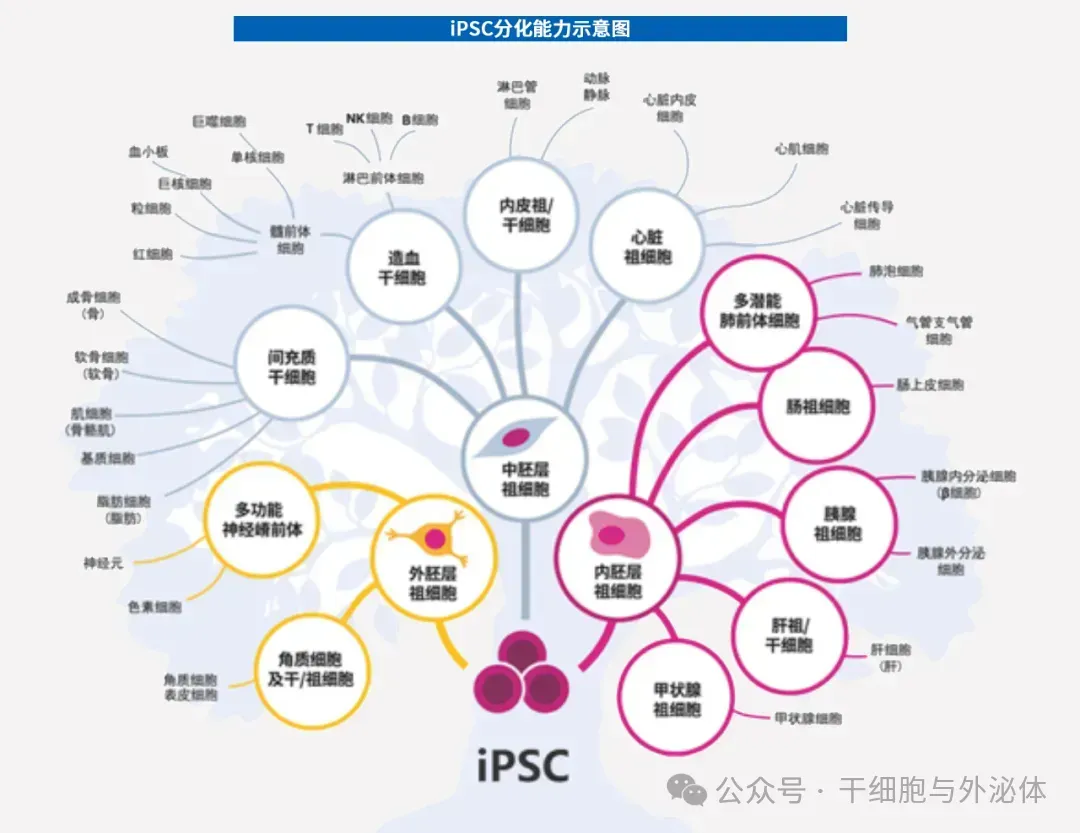
Figure: Because iPS cells have the potential to expand infinitely in vitro and differentiate into any cell type in vivo, they can be used to generate a variety of differentiated cells for cell therapy, disease modeling, drug discovery and toxicology screening.
iPS cells are known as "a great technology that changes the world", and Nobu Yamanaka, the scientist who first discovered iPS technology, won the 2012 Nobel Prize in Physiology and Medicine.
On August 16, 2024, the Future Science Awards Committee officially announced the winners of the 2024 Future Science Awards. In the field of life sciences, Professor Deng Hongkui of Peking University won the "Life Science Award" for his outstanding work in using chemical methods to reprogram somatic cells into iPS cells and change the fate and state of cells. It is reported that the Future Science Award is also known as the "China Nobel Prize", with a single prize of US$1 million (about 7.2 million yuan).
Pei Duanqing, former president of the Guangzhou Institute of Biomedicine and Health of the Chinese Academy of Sciences, once said in CCTV's "I am the Future" program: iPS cells may allow humans to live to 100, 120, 200, or even beyond...
The "seventy-two changes" of inducing pluripotent stem cells have made many diseases no longer worry about!
iPS cells are a new technology that revolutionizes medicine. Clinical trials based on iPS cell technology are being conducted in the United States, China, Japan and other countries (e.g., NCT03763136, NCT03759405, NCT04396899, NCT04106167, NCT03841110, and NCT04245722, etc.).
Because iPS cells have the function of "72 changes", they have broad application prospects in the fields of biology and medicine, and have become an important source of cells for implementing regenerative medicine and cell therapy. iPS cell technology opens a new era of stem cell therapy.
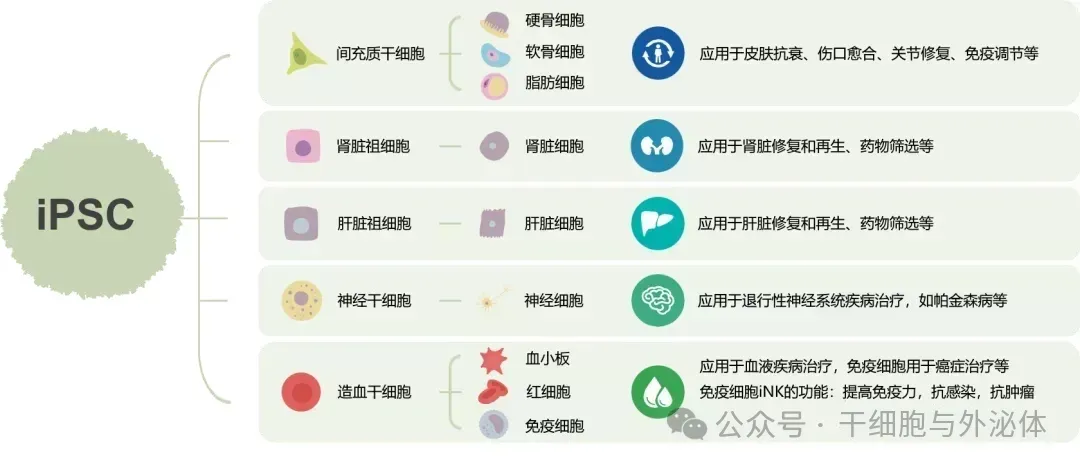
Figure: At present, there have been many studies on the application of iPS cells in disease treatment. It has entered the clinical experimental stage abroad and has great prospects in medical use.
iPS cell technology can achieve large-scale production in vitro, which not only avoids the ethical issues of embryonic stem cell research, but also avoids problems such as limited differentiation potential of autologous pluripotent stem cells and immune rejection after transplantation. iPS cells have demonstrated their infinite potential in the field of disease.
As early as 2007, scientists Hanna and others used hematopoietic precursor cells obtained by iPSCs technology to successfully cure sickle cell anemia; in 2009, scientists Soldner and others successfully induced human iPSCs from which foreign genes were removed into dopamine neurons, making an important contribution to the treatment of Parkinson's disease; in the same year, researchers used iPSCs technology to successfully cure hemophilia.
Cell therapy derived from iPS cells has become one of the mainstream development directions in this field due to its unique advantages. Among them, nearly 35 domestic companies have deployed the iPS cell field, and as the global cell therapy market continues to expand, as an important direction of cell therapy, it is expected to be demonstrated in future commercialization with its high accessibility advantages. It has great potential and occupies a certain share of the market.
World's first iPS cell treatment for spinal cord injury
On February 18, 2019, the Japanese government approved a clinical research application from Keio University in Japan on transplanting neural stem cells induced by iPS cells into patients with severe spinal cord injury. This clinic recruited 4 adult patients with severe spinal cord injuries to treat. In this treatment, the researchers will treat it by using iPS cells to transform them into cells that serve as the basis of nerve cells, transplanting about 2 million new nerve cells into the patient's injured area at a time.
iPS cells generate super killer T cells of cancer cells in vitro
In December 2018, a research team at Kyoto University in Japan used iPS cells to successfully cultivate "killer T cells" with the ability to kill cancer cells, making regenerative immune cell therapy a step forward in clinical cancer treatment. In July 2024, in a new study, Professor Shin Kaneko of Kyoto University in Japan and his team successfully induced regulatory T cell (Treg)-like cells from conventional helper T cells (Tcons) derived from iPS cells and demonstrated their ability to inhibit xenograft-versus-host disease (GvHD).
Using iPS cells to repair brain damage
On April 21, 2021, a research team at the University of California, Los Angeles and the University of Chicago used iPS-induced glial cells to inject them into the brain of a mouse model with human stroke and dementia symptoms, successfully repairing it. Brain damage and improve memory function. This study shows that this iPS-based stem cell therapy can activate the brain's self-repair, prevent the progressive development of white matter strokes, and prevent the emergence of dementia.
iPS cells achieve mass production of platelets
In 2017, a Japanese team achieved mass production of platelets using iPS cells. Blood transfusions may become possible without blood! This research was carried out by 16 Japanese pharmaceutical companies, including Kyoto University's iPS Cell Research Institute and Otsuka Pharmaceutical, which undertook manufacturing, separation, and preservation. Based on the successfully built technology platform for mass production of platelets using iPS cells successfully built by Kyoto University's iPS Cell Research Institute, it can meet the large demand of more than 100 billion platelets required for a single transfusion in practical clinical applications. The research results were published in the international journal Cell on July 13, 2018.
World's first iPS cell therapy for type 2 diabetes
Diabetes is a worldwide problem, and my country has as many as 140 million "sugar people", which is the country with the largest number of diabetic patients in the world. Among them, about 40 million need to rely on insulin injections for life.
Getting rid of insulin dependence and realizing complete "sugar control" is the ultimate dream of diabetic patients, and now, the development of stem cell technology is bringing this dream into reality.
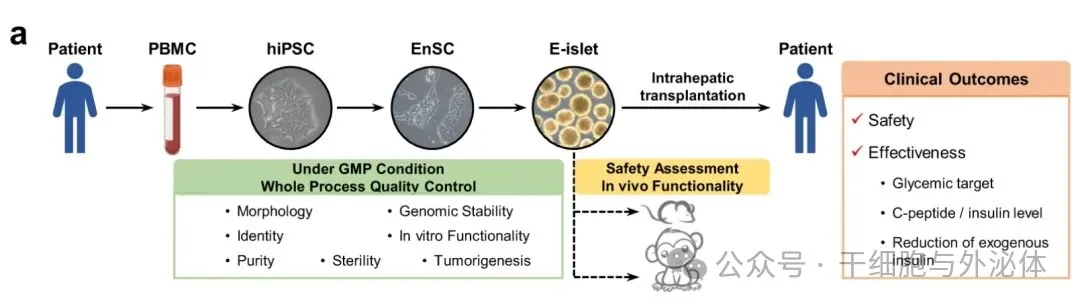
Picture: It is reported that the first beneficiary was a 59-year-old man with a 25-year history of type 2 diabetes and had developed end-stage diabetic nephropathy (uremia). In June 2017, he developed end-stage diabetic nephropathy and received a kidney transplant. However, because its islet function is almost exhausted, multiple insulin injections are required every day, and there is a great risk of serious diabetes complications in the future.
In April 2024, the Cheng Xin Research Group of the Center for Excellence and Innovation in Molecular Cell Science, Chinese Academy of Sciences, and the Yin Hao team of Shanghai Changzheng Hospital of Naval Medical University published the latest research results in the international academic journal Cell Discovery: <Treating a type 2 diabetic patient with impaired pancreatic islet function by personalized endoderm stem cell-derived islet tissue>. This is the world's first autologous regenerative islet transplantation using iPS cells, allowing a diabetic patient with severely impaired islet function to completely get rid of insulin for 33 months and stop using hypoglycemic drugs. From insulin to cell therapy, my country's diabetes treatment has ushered in a new chapter of healing!
iPS cell-derived mesenchymal stem cells
On May 22, 2024, clinical research teams from Australia and the United Kingdom published important research results titled "Two-year safety outcomes of iPS cell-derived mesenchymal cells in acute steroid-resistant graft-versus-host disease" in the industry journal "nature medicine". This clinical study (ClinicalTrials.gov Registration: NCT02923375), the first completed clinical trial of induced pluripotent stem cell (iPS cell) derived cells, was conducted in 15 participants with steroid-resistant acute graft-versus-host disease.
The world's first! Xiangya Hospital completes administration of iPS cells to clinical subjects with cerebral infarction
In January 2024, Professor Yang Zhiquan, director of the Functional Neurosurgery Department of Xiangya Hospital of Central South University, conducted the world's first clinical subject administered iPS cells to treat cerebral infarction, and completed 21 days of observation. The patient's physical indicators were normal. He was discharged smoothly 3 days after surgery.
The patient had a large cerebral infarction for 2 years, resulting in hemiplegia and weakness of hands and feet, which could not be cured by many treatments. Yang Zhiquan provided patients with the opportunity to participate in the "Dose-escalation, single-center, open-label clinical study to evaluate the safety and tolerability of human induced pluripotent stem cell-derived forebrain nerve precursor cell injection (hNPC01 injection) in improving the sequelae of hemiplegia in ischemic stroke", hoping to successfully cure the phenomenon of cerebral infarction hemiplegia.
In addition, in May 2019, cardiomyocytes differentiated from iPSC were injected in Nanjing Gulou Hospital. The operation was completed by Professor Wang Dongjin, director of the cardiothoracic surgery department of the hospital. This is the world's first known clinical application of iPSC technology to treat damaged hearts.

Induced pluripotent stem cells not only have the seventy-two transformations of "Sun Wukong", they also have more superpowers
It can be said that from the birth of iPSC technology in 2006, to the establishment and improvement of its technical system, to its extensive application in life sciences and medical research, iPSCs technology has fully demonstrated its strong contribution to the development of life sciences and medicine. Promote the role, a milestone. Unlike embryonic stem cells and pluripotent stem cells, iPSC breaks through the limitations of ethical issues and has great potential and bright prospects in disease modeling, drug development, and regenerative medicine.
In addition to the clinical end, iPSC also has two major application directions: for pharmaceutical companies, providing drug screening CRO services; for scientific research, establishing disease models.
1. Drug Screening CRO Services
Traditional drug screening is mainly based on ordinary cell models or animal models, but there are limitations such as inconsistent model and pathology, high cost, and long cycle. iPSC has the advantages of infinite proliferation, directional differentiation, and highly reduced pathological states, and is applied to drug screening. Can improve efficiency and reduce costs.
Specifically, in the early stages of drug development, drugs with high elimination rates are usually related to inaccurate sub-optimal screening test results in vivo. At present, the obstacle to improving the efficiency of new drug research and development is that non-human animal models are used to evaluate the target organ toxicity of drugs, and human toxicity cannot be accurately predicted and evaluated.
A 2019 study showed that about 1 in 25 to 1 in 4 users of the top ten drugs with the highest sales revenue in the United States benefited, raising new questions: whether current clinical trials have tested enough individuals to characterize the properties of compounds on a broad human scale.
Recently, legislators and the FDA in various countries have recognized the problems inherent in animal models and taken steps to address them. In 2022, the Biden administration passed the FDA Modernization Act 2.0. This regulation allows for the use of specific experimental methods in place of animal testing, including cell-based tests such as human induced pluripotent stem cells (IPscs), organoids and microorgan-on-a-chip (OoCs), and advanced artificial intelligence (AI) methods such as generative adversary-networks (GANs) and language models. Currently, these alternative trials can seek FDA waivers to evaluate the efficacy and safety of preclinical drugs. The bill marks a major shift from allowing preclinical studies to use the latest scientific techniques to predict human responses to drugs, relying solely on increasingly outdated animal tests.
Ipscs, for example, offer the possibility of overcoming these obstacles by displaying many of the same characteristics as normal cells in vivo, such as morphological structure, gene expression, functional ion channels, receptor expression, and electrophysiological properties. These characteristics all support the use of IPSC-differentiated cardiomyocytes for drug cardiotoxicity detection.
What's more, large-scale drug screening usually requires a lot of cells. Because IPscs can proliferate indefinitely in vitro, disease-model IPscs as well as differentiated functional cells are available indefinitely, and the consistency and stability of cells from batch to batch can be guaranteed when prepared industrially.
2. Establishing disease models
iPSC technology can reprogram a patient's somatic cells into patient-specific iPSC, which has clear disease correlation and genetic information, and can further induce differentiation into disease-related cell types or organoid models, thereby building an in vitro disease model.
For example, the research team at the Graduate School of Okayama University in Japan used the culture supernatant of a human liver cancer cell line that secretes a variety of inflammatory substances to culture mouse iPSC, induced the iPSC into tumor stem cells, and then transplanted the tumor stem cells into the liver of nude mice to successfully create a liver cancer model. This is the first time in the world that a liver cancer model has been successfully produced.
In short, induced pluripotent stem cells (iPSC) have shown great application potential as an important clinical treatment and scientific research tool. From personalized treatment of diabetes to research on various complex diseases, iPSC opens new doors for us to explore the secrets of life. We look forward to more scientific research results and clinical applications in the future to contribute more to the cause of human health. In the near future, iPSC-based cell therapy technology will become a true clinical option, bringing good news to patients on a global scale.