
Induced pluripotent stem cells (technology) are a pot of boiling Mengpo soup that can make you forget every bit of the differentiation path of cells in your previous life. Let the cells reincarnate and return to the starting point, with infinite possibilities.
1. Start with two concepts
To fully understand induced pluripotent stem cells, we must first understand two concepts: "reprogramming" and "induced pluripotent stem cells."
concept 1
Cell reprogramming: refers to changes in gene expression patterns within cells, which are ultimately reflected in changes in the biological characteristics of cells, such as dedifferentiation of differentiated cells, or direct transdifferentiation of differentiated cells.
Concept 2
Induced Pluripotent Stem Cells: Some genes (originally Oct3/4, Soc2, c-Myc and Klf4) are introduced into ordinary body cells such as skin through a retroviral vector, and they are "initialized" to enable them to function as stem cells. These are "iPSCs".
"iPSCs" are not only very similar to ES cells in terms of cell morphology, growth characteristics, and expression of stem cell markers, but are also almost identical to ES cells in terms of DNA methylation patterns, gene expression profiles, chromatin status, and formation of chimeras.(Embryonic stem cells).
Technical route:
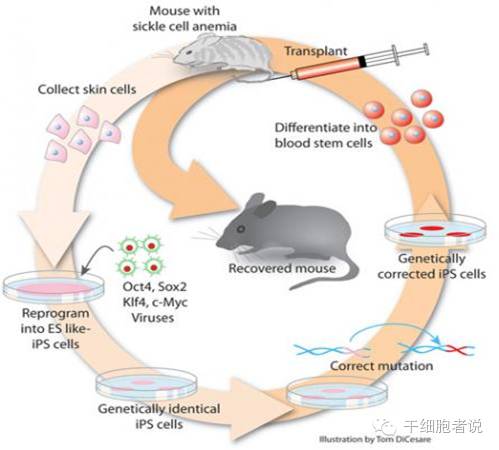
2. The story begins here: the present life of induced pluripotent stem cells (iPSCs)
As we all know, the nucleus of every cell contains almost all the genetic information of this organism (of course, a very small amount is stored in the cytoplasm). This genetic information determines what kind of individual can develop into. This is: "The totipotency of the cell nucleus."
For each cell, its DNA sequence is the same, but the patterns, structures and modifications (chemical modifications such as methylation, acetylation, phosphorylation and glycosylation) of its DNA, chromosomes and nuclear proteins are different, and these differences lead to different cell fates. These differences allow some of them to differentiate into multiple cells, while others can only perform one function.
During the development of higher organisms, changes in DNA, chromosomes and nuclear proteins are basically an irreversible programmed process. Just like a baby, it will continue to grow up after birth, becoming children, teenagers, then youth, adulthood and old age. However, scientists were just whimsical and wanted to get "rejuvenated" cells, so the technology of "reprogramming" came into being.
The earliest reprogramming was implemented on the amphibian-frog. In May 1952, Robert William Briggs and Thomas Joseph King of Philadelphia, Pennsylvania, published an article in the Proceedings of the National Academy of Sciences. After they successfully removed the nucleus of a frog oocyte, the nucleus of an adult cell of a frog was transplanted, and the oocyte was cultured to develop into a complete individual. This is the world's first animal cloning, and the subsequent "Dolly" sheep was completed based on this work. The principle is exactly the same, but the process is much more complex and arduous. The higher the animal, the more troublesome the process will be.
However, this reprogramming method for nuclear transfer is very cumbersome, requiring precise professional equipment and highly skilled technicians. The most troublesome aspect is the need for eggs as recipients. So scientists are always brainstorming how to reprogram cells without using eggs or undergoing nuclear transfer?
In 2006, Japanese scientist Shinya Yamanaka and his beloved apprentice Kazutoshi Takahashi successfully cultured mouse "iPSCs" in vitro for the first time, realizing the reprogramming of non-nuclear transplantation of somatic cells for the first time. This is an epoch-making discovery, and many people are guessing when this work will win the Nobel Prize. Later, sure enough, the research won the 2012 Nobel Prize in Physiology or Medicine.
In fact, the design of his work is very simple. It is to select 24 genes that are highly expressed in embryonic stem cells, use a virus system to do transgenic operations, let them be specifically expressed in adult cells, and then see if they will cause What changes have occurred to the cells? Then they subtracted and removed them in turn and carried out transgenic operations. Finally, they screened out four genes: Oct4, Socx2, Klf4 and c-Myc, and found that after they were transferred together into mouse fibroblasts through retroviruses, Fibroblasts can be induced to return to the state of young embryonic stem cells.
The laboratory of Professor James Thomson of the University of Wisconsin, the most famous embryonic stem cell laboratory at that time, was also doing similar work. They also selected 24 genes and were also trying to test which permutations and combinations could get what kind of results, but they were doing it in human cells, while Nobu Yamanaka and his others were doing it in mouse cells. In this case, a staff member in a small laboratory can easily have the idea: Can we beat them when all the big scientists are doing it? Perhaps Shinya Yamanaka once thought about this, but in the end he persisted and succeeded.
3. Advantages of induced pluripotent stem cells (iPSCs)
Ethical advantages
iPSCs are undoubtedly the most important discovery in stem cell science in recent years, so much so that they won the Nobel Prize in just six years. Its advantages are self-evident.
Its biggest advantage is that it avoids the ethical barriers of embryonic stem cell research and uses adult cells to cultivate embryonic stem cells without involving issues such as embryos. Secondly, it also avoids the problem of donating donors and recipients. iPSCs technology can completely use your own cells to treat your own diseases. You don't have to ask for it, as long as you want it. With iPSCs, it is so willful!
immunodominant
The immune advantages of iPSCs are self-evident. In theory, there should be no immune rejection.
However, some articles pointed out that because iPSCs are cultured for multiple generations in vitro, there will be a process of re-differentiation before they are used for treatment. From adult cell de-differentiation, to in vitro culture, and then to re-differentiation, it is difficult to guarantee that there will be no immunogenicity changes. Especially for immune cells, there is also the problem of immune rearrangement, so we cannot assume that autologous iPSCs are not immune rejected. But if we consider it from the perspective of immunogenicity alone, iPSCs should be the first choice.
individual advantages
Similarly, iPSCs can provide you with "personalized services" because you can use your own cells to screen for drugs. It is more reasonable to do so: there are many types based on many diseases, and the genotypes based on the same disease may vary widely. We can establish cell banks of various types through iPSCs technology, and then use these cell banks to screen drugs. The main advantage is that cells are easier to obtain and cultivate. Because adult cells are difficult to subculture for a long time in vitro, iPSCs are a type of embryonic stem cells that can theoretically be subcultured indefinitely and are also cells that are easy to manipulate genetically.
At the same time, we can also study how these diseases develop through the redifferentiation process of these cells, which genes and factors cause these diseases, and what the relationship between them is, which also provides us with the treatment of these diseases. basis. Using iPSCs technology, we can trace the state of cells back to the early stages of embryonic development and study the disease from its original source. It is especially suitable for studying diseases with genetic factors.
technical advantages
Another advantage of iPSCs is their technical advantages. iPSCs technology is not only mature and simple, but also relatively cheap: traditional nuclear transplants require very skilled experienced workers, complete laboratories, and high-quality experimental animals. A microoperated microscope alone costs millions. How many laboratories can afford this? iPSCs technology has lowered the technical threshold a lot, allowing many ordinary laboratories to try and succeed.
Therefore, once Nobu Yamanaka's iPSCs article appeared, it immediately set off a wave of induced pluripotent stem cells in the world. Since the only iPSCs article in 2006, more than 3000 articles have been published so far, and follow-up research is still in progress. On the one hand, this is because the application prospects of iPSCs technology are very broad, and on the other hand, this technology is simple and easy to implement.
4. Outlook research on induced pluripotent stem cells (iPSCs)
Be yourself-individual-specific stem cells
The most attractive prospect of iPSCs is its "personalized service" feature. From a biological point of view, no two people in this world are exactly the same. Even identical twins with the same genotype may have different epigenetic characteristics. Differences in the environment of two people's encounters in life can also lead to physiological and pathological differences. Therefore, everyone's illness will not be exactly the same. Forget about a minor illness, but if you get a serious illness, who doesn't want to get the most effective treatment in the shortest possible time? But in fact, in today's medicine, the "trial and error method" is still often used-first apply medicine and see if it works, it will be the disease, and if it does not work, it will be ruled out-this is called "diagnostic treatment."
Of course, with personalized iPSCs, it wouldn't be possible to use our own cells to repair our own tissues.
Science fiction films (such as "Escape from Clone Island") often include scenes of dog blood using "human clones" as a "backup" to provide healthy organs and tissues for humans. In fact, there is no need to be so troublesome at all. Why should we cultivate a complete "human"? Wouldn't it be enough to have universal cells? iPSCs are our universal cells. However, so far, we have not been able to directly cultivate a complete organ using "universal cells" in vitro.
Change if you want--directional reprogramming
iPSCs have their limitations. Like embryonic stem cells, they are tumorigenic. Therefore, there is another option, which is "transdifferentiation", that is, directed reprogramming.
iPSCs restore terminally differentiated cells to their state of embryonic stem cells. Can we directly reprogram terminally differentiated cells into another type of cell, either adult stem cells, or another type of terminally differentiated cell?
When iPSCs technology was promoted,"transdifferentiation" technology ushered in its own "spring". In 2010, three articles were successively published in the top international journals "Nature" and "Cell", which dealt with the transformation of fibroblasts into nerve cells, blood progenitor cells and cardiomyocytes. They all used fibroblast cells, one of the most commonly used cells in iPSCs, to induce and cultivate functional nerve, blood and myocardial cells. This is a considerable achievement and an important advancement in regenerative medicine.
Resurrection in situ-fixed-point reprogramming
It has been suggested that we can use reprogramming technology to reprogram other types of cells in damaged tissue areas into functional cell types that we need. Does this sound science fiction? But this is not impossible, because this technology can be based on another technology already under development-individual-level genetic modification technology. Some people have used adenovirus to do such work for a long time. For example, in 1996, adenovirus was used to treat ornithine carbamoyltransferase deficiency, and it has entered the first-phase clinical trial. It's just that no one has applied it to induced pluripotent stem cell technology or transdifferentiation technology.
However, this is still in the imagination stage, and specific implementation still requires hard work.
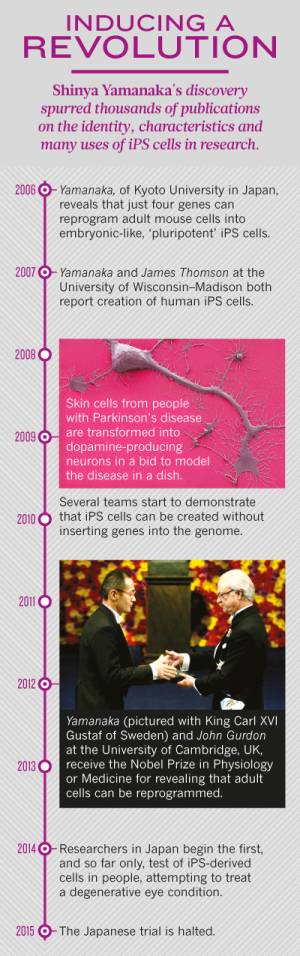
The way forward for iPSCs over the past decade
5. Clinical progress of induced pluripotent stem cells (iPSCs)
In 2013, Dr. Takahashi's team extracted skin cells from two patients with age-related maculopathy and transformed them into retinal pigment epithelium cells (RPE) that can be used for treatment. In September 2014, she led researchers from the Japan Institute of Physics and Chemistry to cultivate a retinal pigment epithelium cell layer using iPSCs and successfully transplanted it into the right eye of a 70-year-old female patient. This was the world's first transplant using iPSCs. surgery.
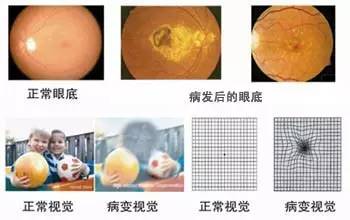
Situation of patients with ocular maculopathy
However, when they were preparing for a second operation, they accidentally discovered two minor genetic mutations in the patient's iPSCs and RPE cells. Although there is no evidence that this causes cancer, treatment has been suspended for patient safety. This stopped other scientists studying iPSCs at the time, and everyone was observing how this research would develop.
Ten years later, the goals of iPS cell research have changed, partly because of limitations in their use for therapy. The only clinical trial of iPS cells came to an abrupt end in 2015 after treating a patient once.