
As a new frontier of medicine, cell and gene therapy is showing great potential and development momentum. Especially in the treatment of refractory diseases, more and more clinical trials of cell and gene therapy are being opened. According to a notice issued by the General Office of the Ministry of Industry and Information Technology, we will speed up the layout and construction of a number of pilot verification platforms that are urgently needed for high-quality development of the manufacturing industry, and the cell and gene therapy industries will benefit greatly. According to incomplete statistics, 9 more CGT therapies were approved for INDs in September 2024, which are organized as follows:
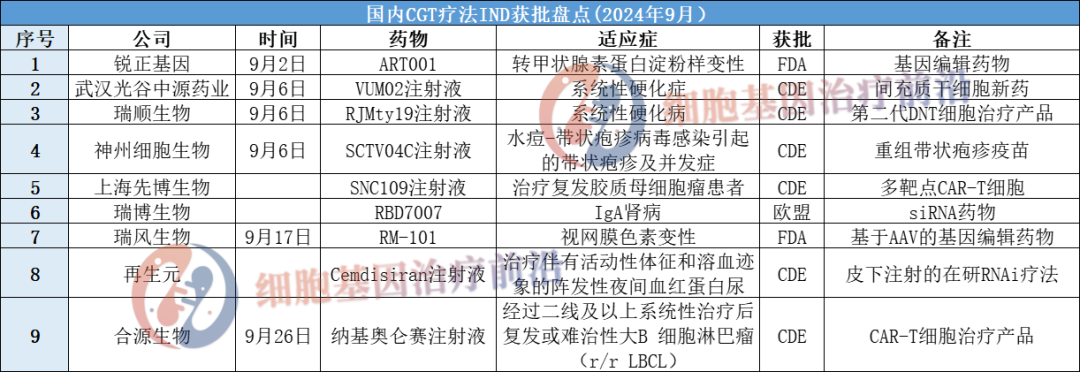
01 Ruizheng Gene: ART001
ART001 developed by Ruizheng Gene has obtained clinical trial approval from the US FDA, becoming my country's first in vivo gene-editing drug based on non-viral vectors to obtain clinical trial approval from the US FDA. The approval of the U.S. FDA IND also makes ART001 the only product of its kind in the world that has received clinical trial approval in China and the United States.
ART001 is the first in vivo gene-editing drug based on non-viral vectors to enter human clinical trials (IIT) in China, and is currently conducting clinical phase 1 trials in China. According to publicly available information, ART001 is currently the only in vivo gene editing product in China that achieves 90% TTR knockdown.
02 Wuhan Optics Valley Zhongyuan Pharmaceutical: VUM02 injection
Wuhan Optics Valley Zhongyuan Pharmaceutical Co., Ltd., a wholly-owned subsidiary of Zhongyuan Xiehe Cell Genetic Engineering Co., Ltd., received the "Drug Clinical Trial Approval Notice" approved and issued by the State Food and Drug Administration on the use of VUM02 injection for the treatment of systemic sclerosis. VUM02 injection (human umbilical cord derived mesenchymal stem cell injection) is a self-developed cryopreserved stem cell preparation. It is a new drug for human umbilical cord derived mesenchymal stem cells (UC-MSC) prepared from screened healthy neonatal umbilical cord tissue through in vitro isolation, expansion, harvest, and cryopreservation. Clinical indications are proposed to increase the treatment of systemic sclerosis.
VUM02 injection for the treatment of idiopathic pulmonary fibrosis was granted orphan drug qualification by the FDA in August 2023, and the first subject was enrolled and administered this year.
03 Ruishun Biotech: RJMty19 Injection
RJMty19 injection (the main active ingredient is CD19-CAR-DNT cells) is a global innovative off-the-shelf universal second-generation DNT cell therapy product developed by Guangdong Ruishun Biotechnology Co., Ltd. based on the global patent of humanized CD19-CAR. Compared with the 11 autologous CD19-CAR-T cell therapy products currently on the market around the world, CD19-CAR-DNT cells derived from healthy donors have many advantages such as standardized preparation, low preparation costs, and instant availability for patients. There is huge potential for market competition in the future, which can fundamentally change the current disadvantages of the commercialization of autologous CAR-T cell products. The safety and effectiveness of this product have been initially verified in recently completed investigator-initiated clinical trials.
04 Shenzhou Cell Biology: SCTV 04C injection
On September 6, Beijing Shenzhou Cell Biotechnology Group Co., Ltd. issued Announcement No. 2024-026 announcing that it had received the "Drug Clinical Trial Approval Notice" approved and issued by NMPA, and agreed to the self-developed recombinant shingles vaccine product SCTV04C injection. Carry out prevention of varicella-shingles virus in healthy adults Clinical trial on herpes zoster and complications caused by (Varicella-Zoster Virus (VZV)) infection (Acceptance Nos.: CXSL2400396, CXSL2400397). Herpes Zoster is an infectious skin disease caused by the reactivation of the latent chicken-zoster virus (VZV). SCTV04C injection is a recombinant protein vaccine independently developed with the goal of differentiated competitive advantages.
05 Shanghai Xianbo Biotech: SNC109 Injection
SNC109 injection is a dual CAR-T cell therapy independently developed by Xianbo Biotech based on the BiTE CAR-T platform. Previously, the product received an official reply from the US FDA on January 23, 2024. SNC109 injection was granted orphan drug status for the treatment of malignant glioma, and on December 26, 2023, it received clinical implied approval from China CDE. The CAR-T cells carry two CAR molecules on their surface that target IL13R α2 and HER2, respectively, which are expressed in glioblastoma. At the same time, this CAR-T therapy can also secrete multi-specific antibodies (BiTE) that target EGFR and EGFRvIII. The BiTE molecule consists of two nanobodies (VHH) and a single chain variable fragment (scFv). Connected in series through flexible fusion adapters can activate one's own T cells to exert a killing effect.
06 Ruibo Biotech: RBD7007
In September, RBD7007, a C5-targeted siRNA drug independently developed by Ruibo Biotech, received EU clinical trial approval and will launch its first human clinical trial in Sweden.
RBD7007 is an siRNA drug targeting C5 independently developed by Ruibo Biotech based on the RIBO-GalSTARTM liver targeting technology platform. It inhibits the expression of C5 protein and blocks the activation of the complement pathway to achieve its role in treating complement-mediated diseases. Preclinical trial data show that RBD7007 has a lasting and powerful inhibition of complement pathway activation, while also having good safety profile, and is expected to bring huge therapeutic benefits to patients with IgA nephropathy and other complement-mediated diseases.
Ruibo Biotech has entered the clinical stage of a number of RIBO-GalSTARTM-based siRNA drugs, including cardiovascular metabolism, liver disease and immune diseases. Ruibo Biotech will continue to cultivate deeply and continue to provide patients with more innovative siRNA drugs.
07 Ruifeng Biotech: RM-101
On September 17, the U.S. Food and Drug Administration (FDA) officially approved Ruifeng Biotech's IND application for RM-101, an innovative drug for Usher syndrome. This approval marks a milestone breakthrough and progress in the development of the world's first AAV-based gene-editing drug for Usher syndrome.
RM-101 is an innovative drug product developed by Ruifeng Biotech to target retinitis pigmentosa associated with the USH2A gene in Usher syndrome. RM-101 is an AAV-based gene editing drug that can specifically target USH2A RNA, regulate alternative splicing biological processes, and induce the restoration of expression of normally functioning proteins. RM-101 is administered by subretinal injection and is expected to achieve one-time administration and long-term effectiveness.
08 Regeneration element: Cemdisiran injection
In September, the official website of the Center for Drug Evaluation (CDE) of the State Food and Drug Administration announced that cemdisiran injection was approved for clinical use in the treatment of adult patients with paroxysmal nocturnal hemoglobinuria (PNH) with active signs and signs of hemolysis. Cemdiran is a subcutaneous injection, small interfering RNA(siRNA) therapy that targets C5 complement.
As early as March 8, 2024, the official website of the Center for Drug Evaluation (CDE) of the State Food and Drug Administration announced that the combination therapy of cemdiran injection and pozelimab injection was approved for clinical use. The advantage of this combination therapy is that it can achieve complete and sustained C5 inhibition at lower doses, reduces the frequency of administration, and facilitates clinical application by subcutaneous administration.