
In 1976, Freidenstein et al. first discovered a type of non-hematopoietic bone marrow stromal cells in bone marrow. These cells are clonal and grow adherent, and are similar in shape to fibroblasts, so they are classified as fibroblast colony forming units. Freidenstein further called them "bone marrow stromal stem cells." In 1992, American biologist Professor Arnold Caplan renamed these cells and proposed a more widely accepted name-"mesenchymal stem cells."
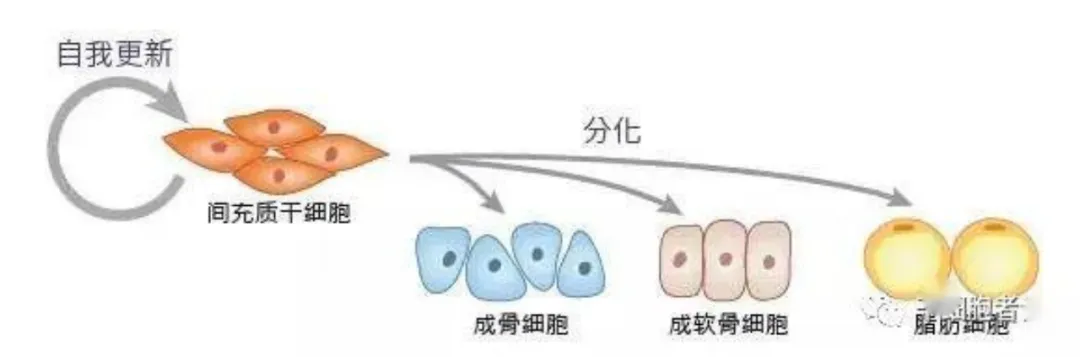
-01-Common sources of mesenchymal stem cells
Mesenchymal stem cells (MSCs) have shown great potential in treating inflammation and degenerative diseases. Such cells not only exist widely in various tissues of the human body, but can also be isolated and identified from these tissues. Mesenchymal stem cells are found throughout almost all tissues, including bone marrow, peripheral blood, umbilical cord blood, placenta, adipose tissue, amniotic fluid, pulp, skin and menstrual blood. In addition, scientists can further expand their application prospects by inducing pluripotent stem cells to differentiate into mesenchymal stem cells.
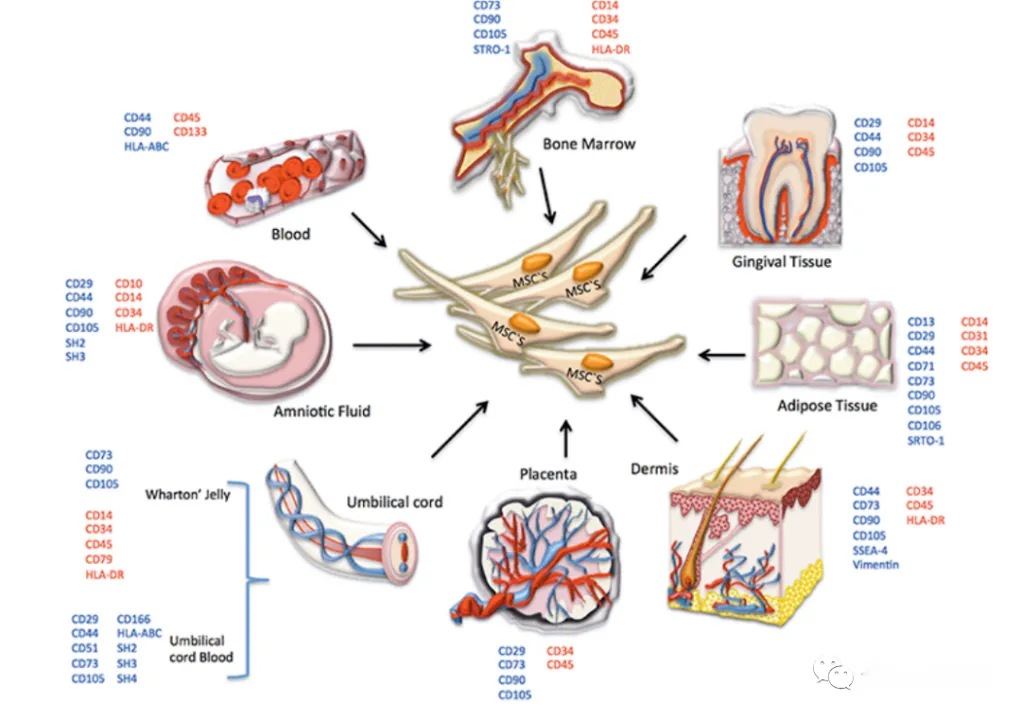
MSCs come from different tissues
-02-China's first iPSC-derived mesenchymal stem cells
On September 8, 2022, the "NCR 100 Injection" from Zhongsheng Traceability Biotechnology Co., Ltd.(referred to as "Zhongsheng Traceability ") was clinically approved. This product is iPSC-derived mesenchymal stem cells (iMSC) and is suitable for the treatment of knee osteoarthritis. This approval marks an important milestone in the field of stem cell clinical research in China. Previously, more than 30 mesenchymal stem cell drugs in China have applied for clinical trials and obtained tacit approval from the State Food and Drug Administration (NMPA). These drugs are all derived from natural tissues (such as umbilical cord, placenta, adipose tissue, dental pulp and menstrual blood) and a kind of mesenchymal stem cells derived from embryonic stem cells (M-021001 cell injection developed by the Institute of Zoology, Chinese Academy of Sciences).

Now, China's first iPSC-derived mesenchymal stem cells (iMSC) clinical trial has finally been approved. Internationally, leading regenerative medicine companies such as Cynata in Australia and Fate in the United States have achieved technological upgrades from natural MSC and NK cells to iMSC and iNK cells. This transformation is mainly due to the fact that iPSC can achieve large-scale production and is more in line with the requirements of pharmacy. At the same time, the clinical effects of natural MSCs and NK cells are not satisfactory.
According to incomplete statistics, 18 mesenchymal stem cell products have been approved for marketing around the world, 10 of which meet the definition of a drug. Among these products, 3 are from Mesoblast in Australia, 4 are from South Korea, and 3 are from Japan and India. The single treatment cost of these mesenchymal stem cell products usually starts from tens of thousands of dollars or even reaches hundreds of thousands of dollars, and the pharmacoeconomics has not yet been fully reflected.
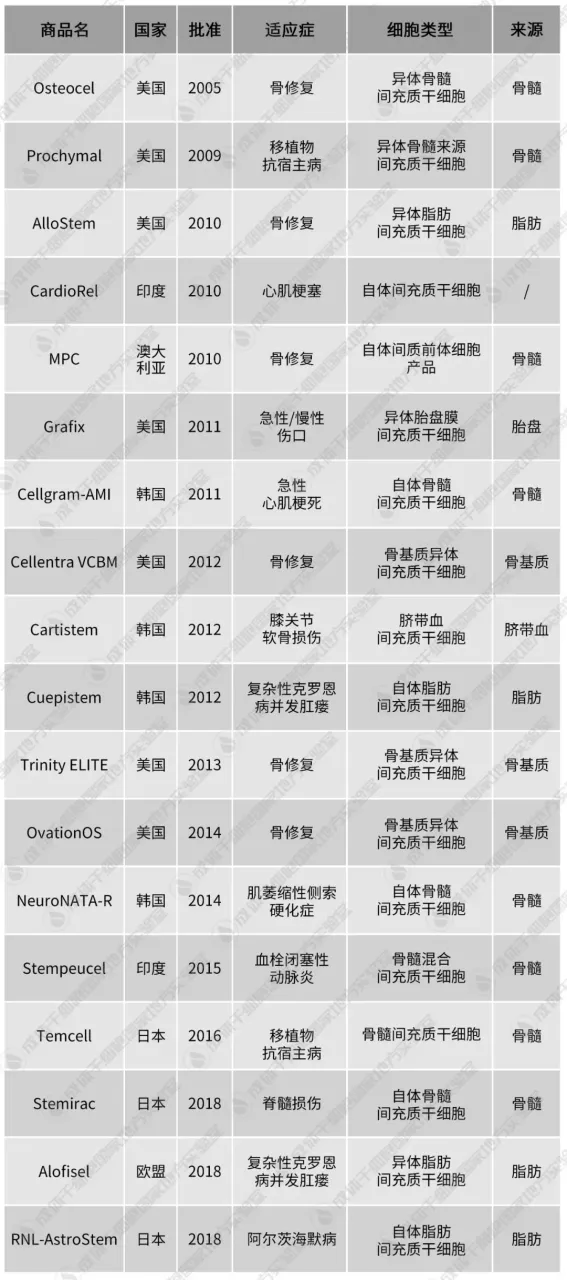
18 mesenchymal stem cell-related products (including orphan drugs, conditionally approved drugs, delisted products, medical products, etc.); the approval date and country are the first approval;Multistem does not count because its official website states that the drug is not a mesenchymal stem cell
-03-Thoughts on the development of iPSC cell drugs
iPSC technology has attracted widespread attention in recent years due to its large-scale production and excellent cell differentiation capabilities, and has become a key project in research institutions in Japan, the United States, Australia and other countries. In the United States, the FDA has approved Fate to conduct a series of clinical trials of iPSC-derived iNK cells and iCAR-T cells. Australia Cynata is also advancing clinical research on its iMSC products, and trials for multiple indications have entered Phase II and Phase III.
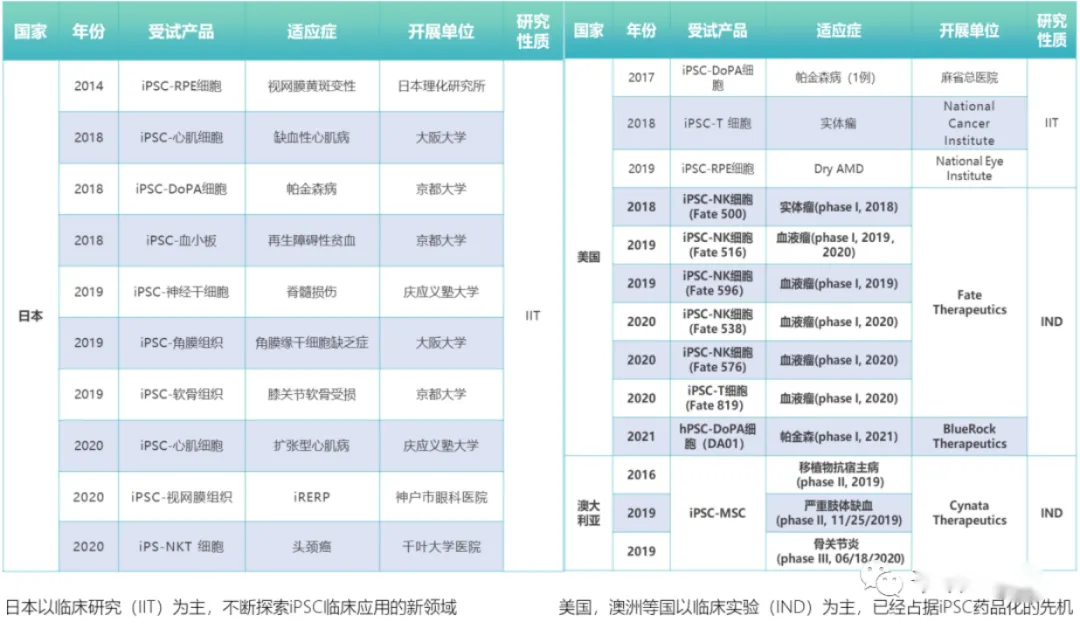
In China, the iPSC-derived mesenchymal stem cell (iMSC) project has obtained clinical implied approval from CDE, marking a breakthrough in iPSC technology in China and also reflecting the support and encouragement of China's regulatory authorities for innovation. However, there are a large number of homogenization applications for domestic CAR-T cell drug registration, resulting in intensive clinical trials and patient shortages, and soaring clinical trial costs, which is undoubtedly a waste of capital and medical resources. In this regard, CDE clearly pointed out in the solicitation draft of the "Guidelines for Clinical Value-oriented Clinical Research and Development of Anticancer Drugs" issued last year: "The highest goal of new drug research and development should be to provide patients with better treatment options, even if the experimental drug is in clinical trials Achieving preset goals cannot prove its actual value to patients, especially when non-optimal treatments are selected as controls."
In layman's terms, this means that when applying for clinical trials in the future, it may be necessary to compare among the optimal treatment options and prove the advantages of the new drug in terms of therapeutic effects. At present, more than 30 items in the domestic drug registration application for natural MSCs cells have received implicit approval, but the indications are often highly similar. Although many of these projects have been approved for clinical trials for many years, experiments have not yet been started or relevant data have been released. This phenomenon reminds us that fierce competition in the field of CAR-T cell drugs is beginning to emerge, and the field of MSCs cell drugs may also face a similar entanglement situation.
-04- concluding remarks
As a relatively "young" technology, iPSC technology shows bright prospects. Its large-scale production and superior differentiation ability make it have huge potential in the field of stem cell pharmaceuticals. It is hoped that relevant fields can make full use of this latecomer advantage, truly realize technological innovation, and avoid the simple "me too" phenomenon. Strictly abiding by national laws and regulations on stem cell clinical research and clinical trials and solidly promoting process optimization are the key to achieving this goal.
In addition to the iMSC field, other iPSC-based treatment directions are also expected to emerge. It is expected that domestic iPSC-related companies can focus on research and development, focus on quality control, and actively cooperate with tertiary A hospitals. Through rigorous and standardized clinical trials, excellent clinical results have been achieved and well-being for human health can be truly realized.