
Human induced pluripotent stem cells (hiPSC) are derived from reprogrammed adult cells and have great prospects in disease modeling, personalized medicine, drug development and regenerative therapies.
Human induced pluripotent stem cells (hiPSCs) are produced by genetic reprogramming of adult cells (such as skin fibroblasts or blood cells) to an embryonic state. When genes encoding specific transcription factors are introduced into adult cells, they acquire key characteristics of stem cell status, namely the ability to differentiate into almost all cell types and self-renew. HiPSC was first reported in Japan in 2007, when Professor Nobu Yamanaka reprogrammed mouse fibroblasts and subsequently human fibroblasts. Because hiPSCs have pluripotent stem cell status, they can provide an unlimited variety of patient-specific cells for research and therapeutic applications.
HiPSC has broad prospects in cell therapy in the field of regenerative medicine and can provide cures or effective treatments for a variety of diseases for which there is currently no feasible treatment method. Importantly, hiPSCs provide a nearly unlimited supply of stem cells because they can differentiate into any type of cell and have the potential to proliferate indefinitely.
The discovery of hiPSC overcomes ethical issues associated with human embryonic stem cells (hESCs), which can be extracted from the inner cell mass of 5-to 7-day-old blastocysts, whose production requires the destruction of human embryos. hESC is considered the gold standard for pluripotent stem cells, but its research is linked to many public and political controversies due to people's different scientific, religious and moral beliefs; in addition, the use of these early embryos has caused a lot of controversy and controversy among pro-life activists and opponents of stem cell research.
In the field of stem cell research, in the early 2000s, human pluripotent stem cells isolated from fetal and adult sources such as amniotic fluid and cord blood were proposed for research and clinical applications because they did not raise any particular ethical issues. However, whether these cells can expand indefinitely and, most importantly, whether they are pluripotent, that is, whether they have the potential to differentiate into any of the three germ layers, remains controversial.
HiPSC combines the best properties of hESC and adult stem cells, avoiding most ethical controversy because no embryos or oocytes are needed to generate hiPSC. It is worth noting that they provide an almost unlimited source of stem cells or immune cells because they can reproduce indefinitely and have the inherent potential to differentiate into any cell type. For these reasons, hiPSC has great potential in disease modeling, personalized treatment, drug discovery and regenerative medicine.
Today, hiPSC can be obtained from a variety of cell sources, including cord blood, peripheral blood, etc. Major advances in reprogramming technology, such as Sendai virus-mediated reprogramming and non-viral methods such as protein and chemistry, have improved the efficiency and safety of hiPSC generation. These methods promote the transformation of somatic cells into pluripotent states, thereby expanding their potential for multiple applications in regenerative medicine and personalized therapy.
Using patient-derived hiPSCs can provide a large number of disease-related cells, even inaccessible cells, such as neurons and cardiomyocytes, for disease modeling to study the causes of human diseases and develop treatment strategies.
In addition, because hiPSC can be obtained from the patient themselves, the rapid development of genome editing technology may allow correction of disease-causing gene mutations in patient-derived hiPSC or the introduction of specific mutations into cells from healthy donors.
hiPSC helps drug development be more effective and safer
Regarding the application of hiPSC in drug development, it is worth mentioning that identifying a successful treatment requires a lot of testing, and the failure rate of drug development even exceeds 90%, so it is a long, high-risk and expensive process. Therefore, the scalability of hiPSC cultures and their ability to replicate endlessly without undergoing replicative aging allow disease phenotypes to be consistently reproduced at culture scale to meet the needs of drug screening.
Animal testing has been a regulatory requirement since 1938 to determine the safety and effectiveness of drugs before they enter clinical trials. Although animal models reveal many disease mechanisms, physiological and genetic differences between animals and humans often lead to failed clinical trials with serious consequences. Between 2007 and 2010, 66% of Phase III failures were due to lack of efficacy and 21% were due to drug toxicity, highlighting the need for improved preclinical assessments and clinical candidate prediction data.
However, recent data shows that this gap in prediction accuracy still exists. A review of 221 animal experiments found that only 50% of the results were consistent with subsequent human studies, and animal testing still failed to predict the toxicity of nearly half of the drugs during Phase 1 trials and early post-marketing withdrawals.
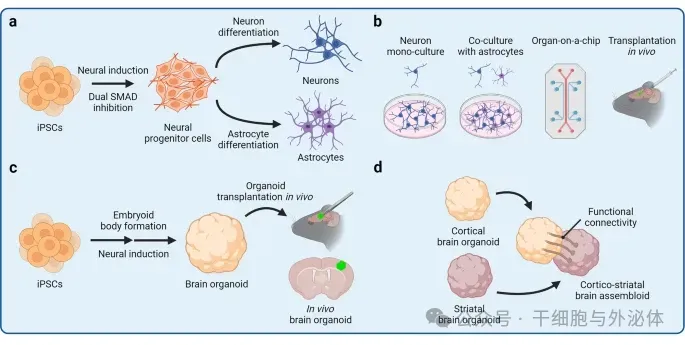
Figure: hiPSC)-derived cell model
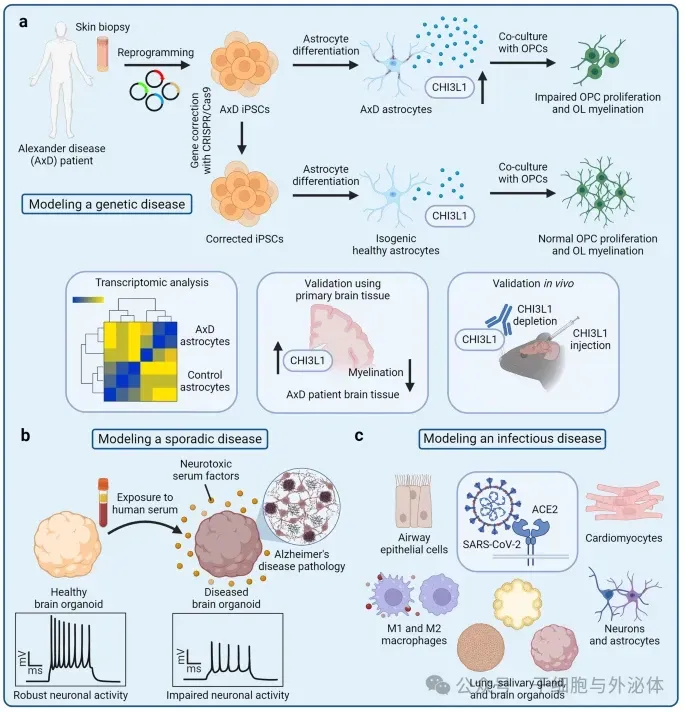
Figure: Disease modeling using hiPSC-derived cells
The FDA Modernization Bill 2.04 was passed by the Senate and House in December 2022, authorizing the use of alternative testing platforms and eliminating the requirement for animal testing for certain drugs before entering clinical trials. The goals are to improve predictability in preclinical safety and effectiveness assessments and reduce the use of animals in drug discovery and development programs.
The new regulations provide significant opportunities for pharmaceutical companies and researchers to explore innovative methods of drug development. It will accelerate innovation and bring safer and more effective drugs to market faster.
A key provision of the FDA Modernization Act 2.0 encourages the use of human-related in vitro models, such as human induced pluripotent stem cells (hiPSC), that can better represent human physiology and disease phenotypes. hiPSCs have become a powerful alternative to animal testing in preclinical drug development because they retain their genetic background and can mimic specific disease phenotypes.
hiPSC technology has facilitated more efficient drug development and has been used in more than 35 clinical trials for neurodegenerative diseases. The use of human biology in preclinical target identification and assessment can improve the accuracy and speed of preclinical drug development, reduce the number of animal trials, and increase the chances of successful clinical trials.
hiPSC cell therapy: From diabetes to cancer treatment
In the field of regenerative medicine, hiPSC is expected to be used to treat a variety of diseases for which there is currently no cure or effective therapy. The basic model of using hiPSCs for cell therapy is to first differentiate them into the desired target cell type, and then transplant the resulting specialized tissue-specific cells into patients.
Cell therapy has recently become a promising approach to repair or replace damaged tissue and design immune responses to diseases such as cancer.
Although primary cells, such as T cells, natural killer (NK) cells and mesenchymal stem cells, can be isolated from patients and then used for autologous cell therapy, other types of cells, such as neurons, cannot be used for transplantation. In addition, the quality of primary cells may be impaired by disease or germline mutations and show unnecessary heterogeneity. Because iPSC can be genetically engineered, clonally expanded, and differentiated into most human cell types, iPSC technology can be used to overcome these limitations.
HiPSC-based cell therapies can be divided into two categories-autologous and allogeneic. In autologous cell therapy, hiPSC comes from the same patient who received the cell transplant. Autologous cell therapy is designed to prevent recipients from immune rejection of the transplant because the immune system recognizes the transplanted cells as "own" tissue. Tissue biopsies are first taken from patients who will receive autologous cell therapy, and the isolated somatic cells are then reprogrammed into hiPSC. These hiPSCs can then be genetically modified to correct undesirable mutations or introduce new gene expression.
In allogeneic cell therapy, iPSC from a universal donor is used for transplantation, avoiding the long and expensive process of producing iPSC from every patient receiving a cell transplant. Desired cells can be differentiated, characterized and stored in advance so that cell products can be provided on-demand or "off-the-shelf" without the need for in-house production.
(1) HiPSC-derived islets functionally cure patients with type 1 diabetes
Recently, a research team from Professor Deng Hongkui from Peking University published their research results in the journal Cell. They extracted cells from patients, used chemical methods to generate hiPSC, grew them into islets, and then injected them back into the patient's abdomen.
Figure: Islet cells in the pancreas
During the procedure, which lasted approximately 30 minutes, the researchers injected the 1.5 million islets they had grown into the abdomen of the first patient, a 25-year-old woman. Place them in your abdomen for easy monitoring and remove them if necessary. Two and a half months later, tests showed that the patient was producing enough insulin to stop the injection.
A year later, she was still producing insulin on her own. The team noted that because of a previous liver transplant, the patient had begun treatment with immunosuppressive drugs; therefore, it was unclear whether her immune system would replicate the type of attack that originally led to her developing type 1 diabetes. The team is currently conducting clinical research on type 2 diabetes.
(2) HiPSC-derived immune cells are used to treat cancer
Cervical cancer is one of the most common malignant tumors in women around the world. Unfortunately, this preventive measure is ineffective against diagnosed cancers, which are often incurable once the cancer metastasizes or recedes.
宫颈癌是全球女性最常见的恶性肿瘤之一。不幸的是,这种预防措施对已确诊的癌症无效,一旦癌症转移或复发,通常无法治愈。
Fortunately, scientists have made significant progress in developing a promising treatment strategy for cervical cancer: regenerative cytotoxic T lymphocytes (rejTs). These lymphocytes can be modified to target HPV-specific antigens that are mainly expressed in cervical cancer cells, thereby constituting a targeted immunotherapy. Ideally, rejTs should be produced by induced pluripotent stem cells (hiPSCs) collected from the patient themselves.
In this context, the research team of Miki Ando, chief professor at the School of Medicine at Shuntangu University in Japan, graduate student Yoshiki Furukawa, and assistant professor Ishii recently made a breakthrough and developed a powerful iPSC-derived rejT for the treatment of cervical cancer. Their research results were published online on December 12, 2023 in Cell Reports Medicine.
Fate Therapeutics, Inc. (NASDAQ: FATE) is a clinical stage biopharmaceutical company dedicated to providing first-class induced pluripotent stem cell (iPSC)-derived cellular immunotherapy to patients with cancer and autoimmune diseases. In May 2024, the company announced preclinical data on iPSC-derived CAR-NK cell candidate products for CD19. FT522 is used to treat relapsed or refractory B-cell lymphomas, including non-Hodgkin lymphoma, follicular lymphoma, etc.
In addition, new advances in hiPSC-derived immune cells have produced powerful iNK and iT cells that have shown strong killing against cancer cells in animal models and clinical trials.
Establish a hiPSC resource library to benefit more people
In principle, hiPSC can be extracted directly from individual patients in need of treatment; this procedure has the advantage of ensuring the same immune status and minimizing the risk of transplant rejection.
However, producing autologous clinical grade cells for individual patients will impose huge time costs and scientific difficulties, and large-scale applications may be economically limited. An alternative to personalized hiPSC therapy is to create a clinic-grade hiPSC resource bank that can be expanded and differentiated for use by a large number of patients.
Ideally, such a library of hiPSC would consist of hiPSCs obtained from healthy volunteer donors of type O blood selected to maximize the chances of human leukocyte antigen matching and thereby minimize the risk of allograft rejection.
Assuming that a single pluripotent stem cell line can provide cells or tissues to a large number of potential recipients, some studies have been carried out in Japan, the United Kingdom and others to calculate how many hiPSC donors are needed to cover sufficient populations in different countries; therefore, cells from a single donor can be frozen forever and can be used by everyone for different cell medical purposes. For example, in Japan, a haploid bank of 27 iPSC lines from 7 donors was established that matched approximately 40% of the Japanese population.
On October 29, 2024, I Peace, inc., a company specializing in human induced pluripotent stem cells (hiPSC) and hiPSC-derived cell therapies. It was announced that a hiPS cell line homozygous for HLA A, C and DPA1 had been successfully generated. Both clone lines are available to cell therapy developers and pharmaceutical companies as GMP and research-grade off-the-shelf products. This cell line is homozygous for HLA A, C and DPA1. More than 100 million people (35.91%) in the United States have this HLA A haplotype.
In short, the discovery of hiPSC has had a profound impact on the fields of cell and gene therapy. From diabetes to cancer treatment, hiPSC has become a valuable source of cells for cell therapy in regenerative medicine.