
At the beginning of this article, we will focus on the preclinical application of iMSC and the research progress of clinical grade iPSC-derived cell products, that is, the pharmacodynamic research data in multiple disease animal models.
iPSC-derived iMSC reflects superior biological properties
Shinya Yamanaka used Sendai virus for the first time in 2006 to transfer a combination of four transcription factors (OCT4, SOX2, KLF4 and c-Myc) into differentiated somatic cells and reprogram them into pluripotent stem cell states. This breakthrough discovery has led different scientists around the world to carry out research on iPSC, thus opening the iPSC era [1]. iPSCs have a wide range of sources and can be obtained from most mature somatic cells including epithelial fibroblasts, adipocytes, renal epithelial cells, nerve cells, islet cells, mesenchymal stem cells, and renal epithelial cells. iPSC retains the epigenetic markers of some donor cells, which may affect cell functions that differentiate in different directions [2-4].
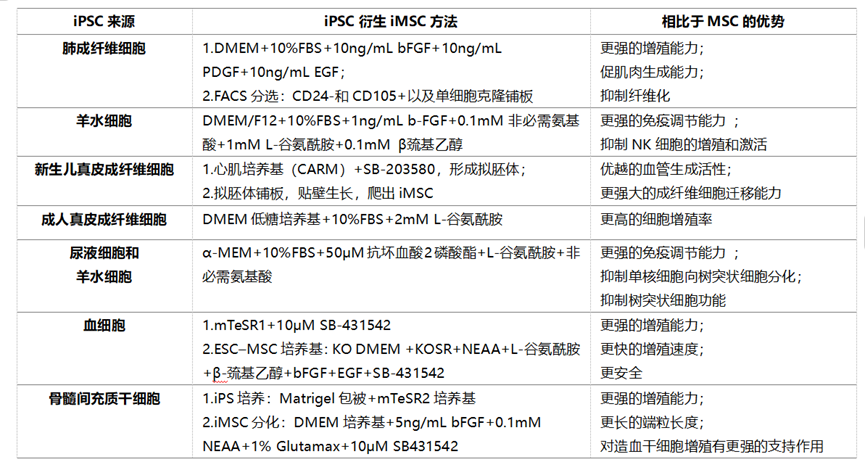
Some researchers compared iMSCs induced by differentiation from three iPSCs derived from gingival, periodontal ligament and lung tissue, and found that the differentiation characteristics of all iMSCs are very similar in terms of pluripotency, surface marker expression, and the differentiation characteristics of the three lines [5]. We use the following table to list the performance characteristics of iMSC cells differentiated from iPSCs from different tissue sources, and found that to a certain extent, iMSC is functionally superior compared with adult MSC.
Table 1. Comparison of the performance of iPSC derived from different tissues and differentiated iMSC [6-10]
Preclinical data from multiple indication models
iMSC has been used in many disease animal models to prove its efficacy. iMSC exhibits similar functions as MSC on tissue regeneration, damage repair, immune regulation, inflammation regulation, etc. Whether iMSC is injected intravenously or locally, the low immunogenicity of iMSC is crucial. Studies have shown that injecting iMSC into the knee joint cavity of nude mice and SD rats with cruciate ligament injury will not cause an in vivo inflammatory response in rats, suggesting the applicability of iMSC xenotransplantation [11].
In terms of immune regulation, infusion of iMSC into the rat GvHD model significantly reduced the strength of T cells, reduced monocyte infiltration after transplantation and the expression of pro-inflammatory factors IFNγ and TNFα in plasma, promoted the expression of IL-10, and significantly prolonged the survival time of the hind limbs of model animals [12]. For inflammatory bowel disease, whether injected into the tail vein or intraperitoneally, it can significantly improve the symptoms of mice, promote intestinal epithelial cell proliferation and intestinal angiogenesis, and iMSC has similar effects to AT-MSC at the same dose [13].
In terms of tissue regeneration, iMSC cell membranes can significantly promote the regeneration of cartilage tissue in nude mice with laryngeal cartilage injury, promote the expression of chondrocyte markers and the deposition of extracellular matrix. In terms of bone regeneration, compared with calcium phosphate particles, the bone parenchyma after local transplantation of iMSC was significantly better, and there was no significant difference in the cortical and central defect areas compared with BM-MSC transplantation [14]. In a steroid-induced rat model of femoral head necrosis, local injection of iMSC prevented bone loss in the necrotic area and promoted cartilage repair [15]. When iMSCs are implanted into a rat periodontal defect model, iMSCs promote periodontal regeneration and the formation of new mineralized tissue [16]. After transplantation of iMSC induced osteoblasts into mice with skull defects, they can support bone formation at the defect site [17].
iMSC is also very effective in damage repair. In rats with cerebral ischemia and reperfusion injury, iMSC can inhibit the activation of microglia at the injury site, and can significantly improve the neural and motor functions of rats in the short and long term [18]. In a mouse renal ischemia reperfusion injury model, different doses of iMSC activated the ERK1/2 signaling pathway, reduced the serum creatinine level and the degree of tubular necrosis in mice, significantly reduced the level of inflammatory cytokines and oxidative stress, and improved renal function [19]. Local injection of iMSC near the myocardial infarction focus can promote the interaction between parenchymal cells and interstitial cells and relieve ventricular remodeling [20]. In mouse models of cardiac dysfunction caused by passive smoking, tail vein injection of iMSC significantly reduced the increase of pro-inflammatory cytokines, restored the expression of anti-inflammatory factors and antioxidant markers, and significantly improved the abnormal shape of the heart [21], suggesting the possibility of iMSC as a cardiovascular treatment drug. iMSC is also widely used for dysfunction. When iMSC was injected into the spleen of rats with liver dysfunction, it was observed that iMSC differentiated into functional hepatocytes that expressed multiple human-specific stem cell markers, promoting liver regeneration [22].
iMSC infusion can also improve serum biochemical indicators in rabbits with renal dysfunction, reduce the degree of renal tissue fibrosis, and restore renal blood flow [23]. In a rat model of erectile dysfunction, local injection of iMSC into the cavernous body can repair damage to penile endothelium and smooth muscle tissue in ED rats, reduce BAX and Caspase-3 levels, and increase Bcl-2 expression, thereby reversing apoptosis and restoring erectile function [24].
Research progress on clinical grade iPSC-derived cell products
As of November 2023, a total of 155 ongoing clinical trials based on iPSC in the international market have been searched on the Clinical Trials website. The indications include multiple myeloma, lymphoma, arthritis, graft-versus-host disease, heart failure, respiratory failure, Parkinson's disease, stroke and other diseases. Among them, a total of 23 clinical trials were conducted for iPSC-derived cell products, and only 3 clinical trials were conducted for iMSC-related.
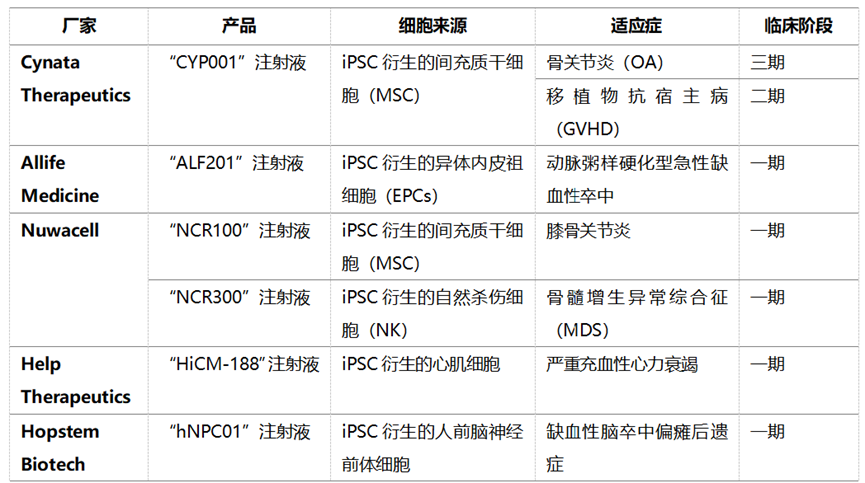
The one that is far ahead in the world is CYP-001 developed by Cynata Therapeutics, with indications for graft-versus-host disease, severe limb ischemia and osteoarthritis. Its clinical phase 1 trial (NCT02923375) recruited 16 subjects with Class II-IV SR-aGvHD. The trial results showed that iMSC is safe and well tolerated without serious adverse events. On the 100th day of iMSC infusion treatment, the OR, CR and OS rates were 86.7%, 53.3% and 86.7%, respectively, demonstrating the huge potential of iMSC products for the treatment of GvHD indications [25].
CAStem (MSC-like cells derived from human embryonic stem cells) developed by Beijing Zehui Biotech is being recruited in a Phase I clinical trial in the United States (NCT04331613). The indications are acute lung injury caused by adult respiratory distress syndrome and viral infection.
The phase I clinical trial (NCT06049342) of the iMSC product (NCR100) traced to Zhongsheng for knee arthritis has been launched and has not been recruited yet. In China, the website of the Center for Drug Evaluation of the State Food and Drug Administration (www.cde.org.cn) shows that five iPSC-derived cell products have been conducted in clinical trials in China, one of which uses iPSC-derived MSC.
Mainly including Chengnuo Medicine ALF201 injection, an iPSC-derived cell drug product developed by Allife Medicine for the treatment of acute ischemic stroke; Alp "Human iPSC-derived cardiomyocyte injection" for heart failure developed by Help Therapeutics; Hoder Bio "hNPC01", an iPSC neural precursor cell product developed by Hopstem Biotech for stroke and craniocerebral injury;"NCR100 injection" developed by Nuwacell for knee osteoarthritis and "NCR300 injection" for myelodysplastic syndrome (MDS), are implicitly approved for clinical trials of NMPA.Table 2. iMSC products under clinical study
Combined with the current preclinical data and clinical trial applications, it can be seen that the enthusiasm of the capital market for iPSC-derived cell products is gradually increasing. The clinical development of most iMSC products is still in the early stages, and the safety and effectiveness of the products are comparable to the current market. The abundant adult MSC treatment pipeline products are comparable.