
In the global competition of iPSC technology, domestic enterprises have demonstrated strong R&D capabilities and market potential. According to incomplete statistics, currently, these 36 domestic enterprises have laid out iPSC-related pipelines.
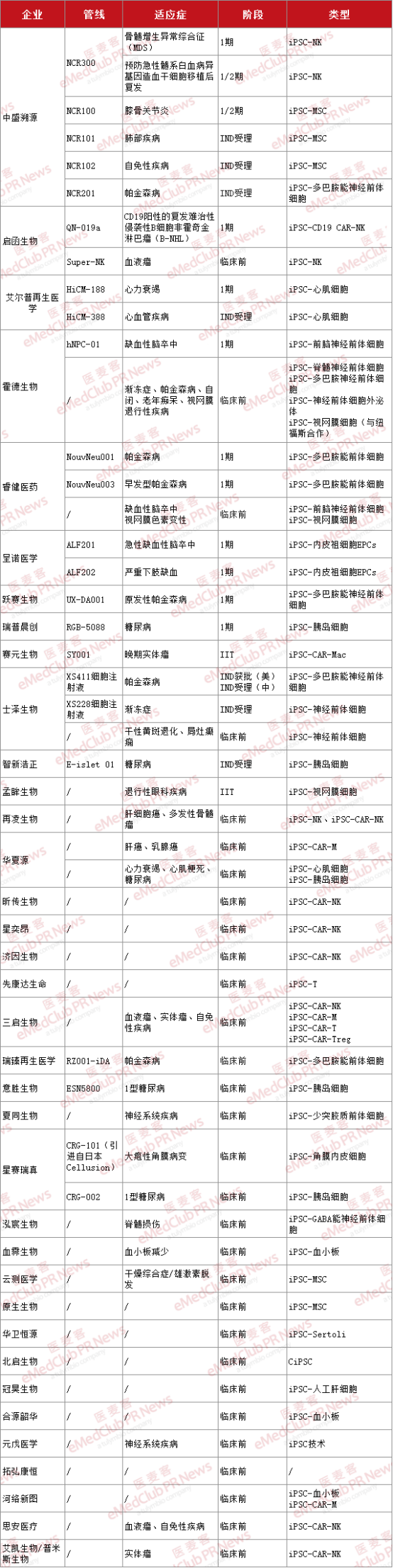
▲ Domestic preclinical and clinical progress of iPSC pipeline enterprises
It can be seen that the R&D pipeline of domestic iPSC enterprises is mainly concentrated in the preclinical stage and early clinical trials (phase 1), which indicates that domestic iPSC technology is still in the key stage of laboratory to clinical transformation, and there is still a certain distance from mature commercial application, and more clinical safety and effectiveness verification is needed.
Up to now, there are 8 domestic companies with iPSC pipeline entering the clinical stage, including Zhongsheng Traceability, Qihan Biology, Elpu Regenerative Medicine, Hode Biology, Ruijian Medicine, Zenuo Medicine, Yue Saibio, Ruipchenchuang. Among them, Zhongsheng Traceability has more pipelines in the iPSC field, covering two major directions: IPSC-derived immune cells and IPSC-derived stem cells.
Although most clinical transformations are still in the early stages, with the support of policies and the influx of capital, we believe that more clinical breakthroughs are expected in the next few years.
From the perspective of indication distribution, the R&D pipeline of domestic iPSC enterprises is mainly concentrated in the two major fields of nervous system diseases and tumor treatment, and also has a layout in the fields of cardiovascular diseases, eye diseases and metabolic diseases.
Neurological diseases are one of the main application areas of iPSC technology, especially in Parkinson's disease, ALS, ischemic stroke and other fields. Taking Parkinson's disease as an example, Zhongsheng traceability, Hode biology, Ruijian Medicine, Yuesai Biology, Shizai Biology, Ruizhen regenerative Medicine and other companies have pipelines for this indication field, among them, Ruijian medicine, Yuesai bio-related pipelines are in the phase 1 clinical stage, Zhongsheng traceability, Shizai bio-related pipeline IND has been accepted. iPSC technology has attracted attention in the field of treatment of nervous system diseases, largely because of the limited therapeutic means for this kind of diseases, and iPSC has the potential to differentiate into nerve cells, which is expected to provide a new therapeutic strategy.
In terms of tumor treatment, it is mainly for some common blood tumors. The pathogenesis of blood tumors is relatively clear, and immune cell therapy has a certain research basis in this field, and the immune cell therapy derived from iPSC technology is expected to bring a new choice for the treatment of related diseases. For this field of disease, the pipelines laid out by two domestic companies, Zhongsheng Traceability and Qihan Biology, are currently in phase 1/2 and Phase 1 clinical trials, respectively.
With the increasing incidence of metabolic diseases such as cardiovascular disease and diabetes, there is a great unmet clinical demand for the application of iPSC technology in these fields. For example, the clinical trial of IPSC-derived cardiomyocytes in the treatment of heart failure led by Epp Regenerative Medicine has achieved positive effects for up to 4 years. Rupchen's IPSC-derived islet cells enable patients with type 1 diabetes to achieve functional healing.
From the perspective of layout pipeline types, the research and development direction of domestic iPSC enterprises is mainly focused on IPSC-derived immune cells and IPSC-derived stem cells, and the preparation of immune cells through iPSC technology can enhance their recognition and killing ability of tumor cells and other target cells, or differentiate into specific cells to repair/replace damaged tissues and organs. To provide new solutions for disease treatment.