
The approval of the listing of two types of stem cells in China and the United States at the end of 2024 has ignited the heat of the stem cell regenerative medicine field, making it a hot topic in 2025. Despite the still cold capital market in the first half of the year, it has risen against the trend. Nearly ten enterprises have completed a cumulative financing of over 500 million yuan, and multiple clinical pipelines have made key progress. The advance charging project in Boao, Hainan has promoted the development of the stem cell therapy industry. The previous article sorted out the basic framework of the stem cell regenerative medicine field. This article conducts a comprehensive review of the domestic pipeline under research for reference and discussion.
First, the ongoing pipeline review shows significant differences in the progress of indications
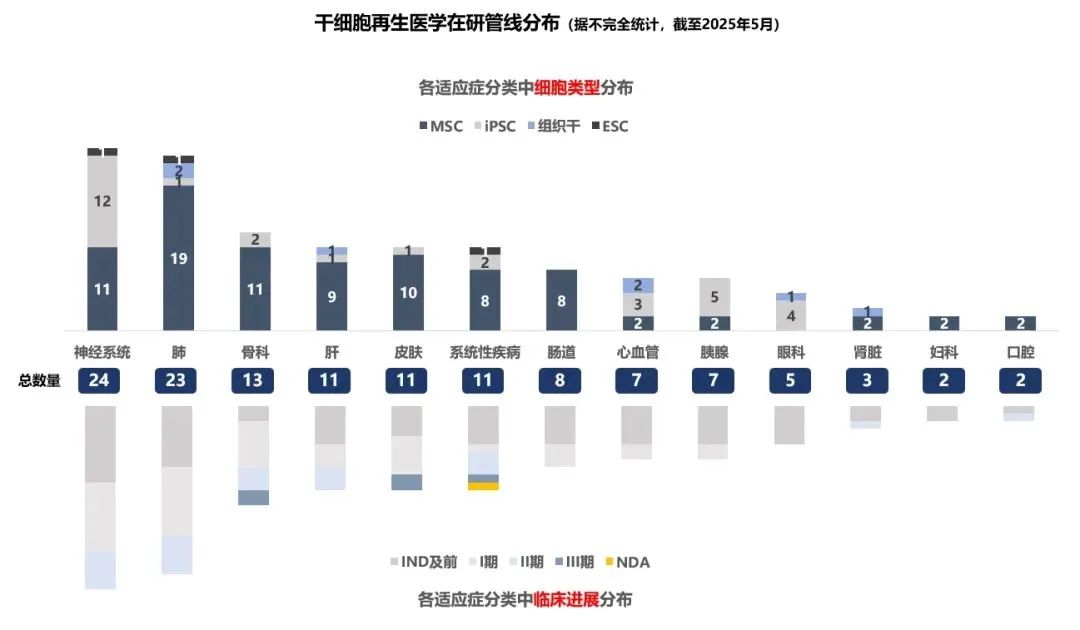
Among the 133 pipelines under research in stem cell regenerative medicine based on public information, from the perspective of indication types, there are as many as 24 pipelines related to the nervous system, such as stroke and Parkinson's disease, with more than half of them following the iPSC pathway. There are 23 pipelines under research for respiratory system indications. At present, there are over 13 pipelines under research for the indication of knee osteoarthritis, and 11 pipelines each for systemic diseases such as liver, skin, and GvHD. Among them, MSC is the main technical approach. In terms of progress, the first stem cell therapy drug to be launched in China currently comes from the human umbilical cord mesenchymal stem cell therapy for GvHD developed by Platinumide. Additionally, several GvHD pipelines are in Phase III or II clinical trials. In addition, the pipelines entering Phase 3 clinical trials include two drugs for knee osteoarthritis indications and two drugs for the treatment of perianal diseases.
From the perspective of cell types, there are over 80 technical pipeline routes for mesenchymal stem cells, and the progress is relatively fast. The first stem cell therapy drug to be launched in China comes from the human umbilical cord mesenchymal stem cell treatment of GvHD by Platinumion Excellence. Additionally, several pipelines are in Phase III or Phase II clinical trials. The number of iPSC pipelines is 35. The progress of these pipelines is relatively early. Only a few pipelines are in Phase I and Phase II clinical trials, while the majority are in preclinical or IND application stages. The number of tissue stem cell pipelines is 7. Among them, the representative Jimeiruisheng's pulmonary tracheal basal layer stem cell therapy for idiopathic pulmonary fibrosis is in clinical phase II/III, while the rest are in preclinical or IND application stages. In terms of embryonic stem cells, the main applications are for Zehui Bio's listing on the Hong Kong Stock Exchange and for Amstein Bio.
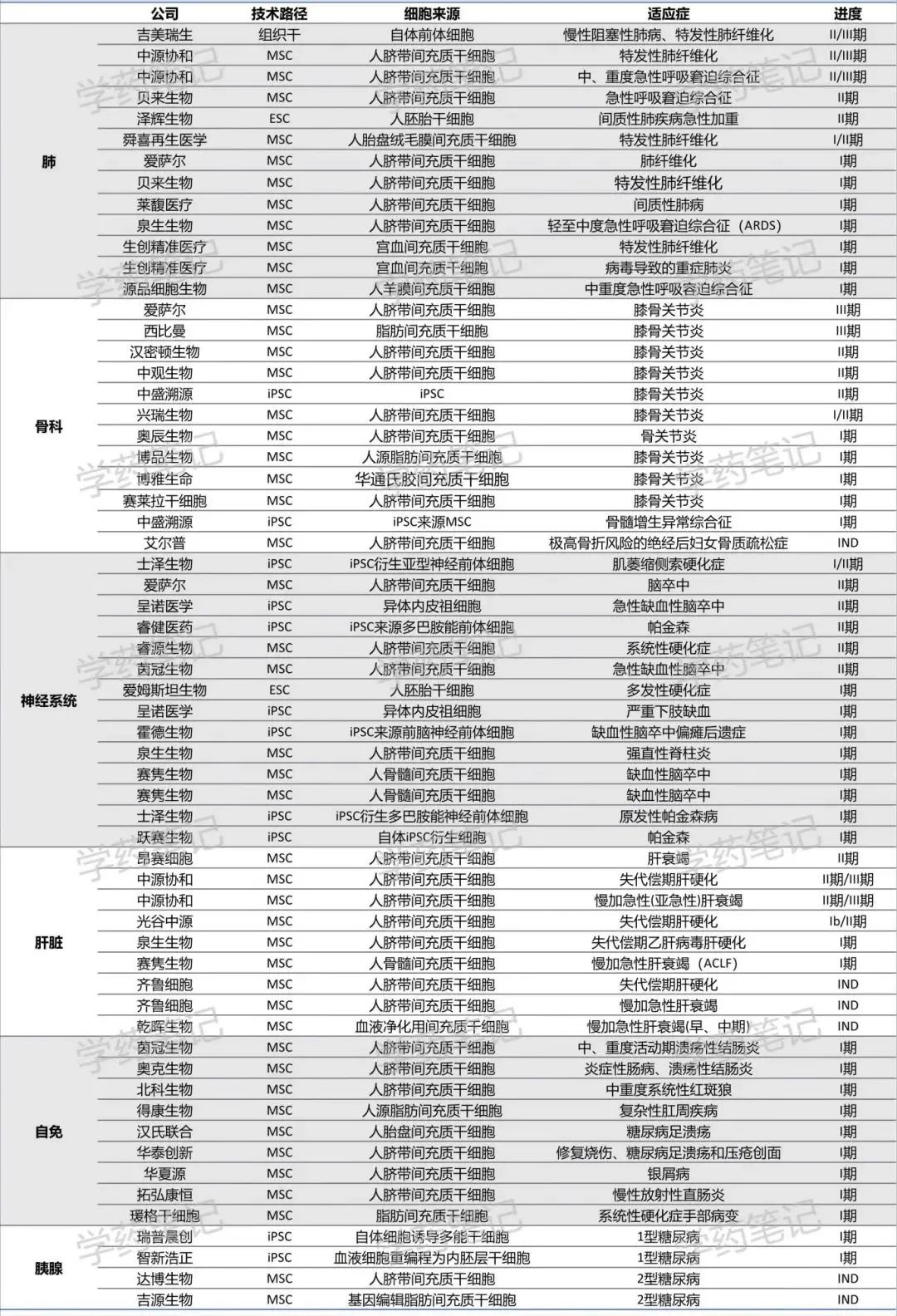
As of May 2025, the situation of some stem cell regenerative medicine pipelines is as follows:
Second, the characteristics of stem cells correspond to different application scenarios
1. Embryonic stem cells
(1) Key features
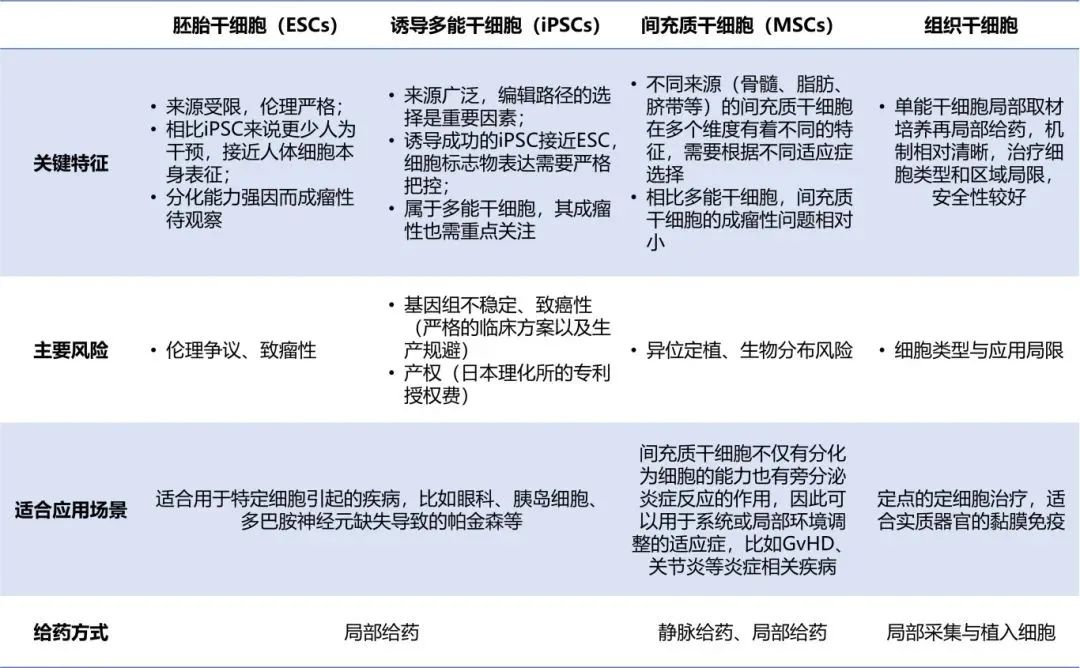
Embryonic stem cells are derived from early embryos and require less human intervention compared to IPscs induced by reverse differentiation. Therefore, they are closer to the characteristics of human cells themselves. However, the sources of embryonic stem cells are limited as their acquisition requires embryo donations that strictly comply with ethical requirements. Therefore, all cell banks used for drug development come from domestic institutions that meet ethical requirements. Currently, the National Stem Cell Resource Bank is the only one in Chinese mainland that provides clinical-grade human embryonic stem cells with ethical information.
(2) Suitable for application scenarios
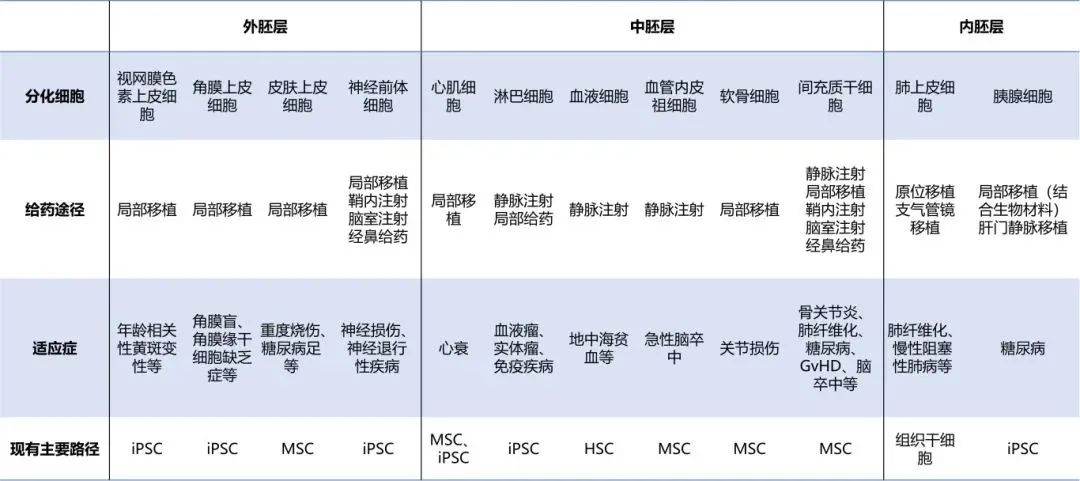
It is suitable for diseases caused by specific cells. According to statistics, it accounts for nearly half of the ophthalmic indications abroad. The unique immune privilege of the eyes, combined with the strengthening of the blood-eye barrier, significantly reduces the risk of rejection of transplanted cells. Moreover, the unique and isolated structure of the eyes minimizes the spread of these cells to other parts of the body, thereby reducing the possibility of unexpected systemic effects. Therefore, it is more suitable for embryonic stem cell therapy.
(3) Breakthrough direction
The issue of safety is the most prominent one. How to ensure that cell transplantation does not cause complications such as tumor formation and teratoma is an important research topic at present. In addition, there are still challenges in the directed induction and differentiation of embryonic stem cells, and different cell lines have different developmental potentials. The pluripotency of embryonic stem cells stems from their unique gene expression regulation mechanism, and the current understanding of this mechanism is still insufficient.
2. Induced pluripotent stem cells
(1) Key features
Induced pluripotent stem cells are cultured by reprogramming and reverse differentiation of human somatic cells, so they have a wide range of sources. Companies can either build their own iPSC cell banks or purchase them from service providers. The criterion for successfully induced IPscs is to be close to embryonic stem cells, so cell characterization needs to be strictly controlled. In addition, as the RiKEN Institute of Japan has relatively complete patent protection related to iPSC, the payment of patent fees is a topic that Chinese iPSC enterprises cannot avoid.
(2) Suitable for application scenarios
It is suitable for diseases caused by specific cells, such as ophthalmic diseases, Parkinson's disease caused by the loss of pancreatic islet cells and dopamine neurons, etc.
(3) Breakthrough direction
1) iPSC reprogramming issue: As iPSC requires multi-step induction in vitro reprogramming, in addition to paying attention to whether the reprogramming method is integrated and the expression efficiency and time, it is also necessary to pay attention to how to establish a more scientific cell characterization method for the reprogrammed cells. iPSC cells may still carry the epigenetic modifications of the original cells after induction. Gene editing may bring many risks, and the quality of their cells needs to be paid more attention to.
2) Rejection issue: For the selection of iPSC and MSC cells, it is necessary to find cell types with higher immune compatibility. In the application projects of iPSC and MSC, allogeneic cells are often used. Universal donor cells can be selected or immunogenic genes can be knocked out. On this basis, immunosuppressants can be selected for clinical combination.
3) Differentiation efficiency: iPSCs require multi-step induction reprogramming. To achieve faster differentiation and minimize the impact of human intervention, as well as to be closer to highly active embryonic stem cells, their differentiation efficiency is particularly crucial.
3. Mesenchymal stem cells
(1) Key features
Mesenchymal stem cells are the first type to be developed into drugs. They not only have the function of differentiation but also play a role in paracrine regulation of inflammatory responses. There are many mesenchymal stem cell banks from sources such as umbilical cord, bone marrow, and fat on the market, making them the most widely studied cell type.
(2) Suitable for application scenarios
Indications that can be used for systemic or local environmental adjustment, such as GvHD, arthritis and other inflammation-related diseases.
(3) Breakthrough direction
1) Tumorigenicity remains to be observed: Although a large number of studies and clinical trials generally report that mesenchymal stem cell therapy is safe and has the least side effects, the possibility of tumor occurrence remains a major issue. This is because mesenchymal stem cells share certain common features with tumor cells, such as prolonged proliferation and high resistance to apoptosis. A variety of factors, including the donor's age and the recipient's immune status, can affect the risk of tumor occurrence after mesenchymal stem cell transplantation. The operation and long-term culture of MSCS may also lead to genetic instability and increase the possibility of malignant transformation.
2) Unclear pharmacokinetics and therapeutic mechanisms: The dynamic distribution, migration efficiency and long-term survival rate of MSCs in vivo remain unclear, which affects dose standardization. Studies have shown that the majority of intravenously injected MSCs reside in the lungs. It is of great significance to enable MSCs to nest to the injured site and exert their functions.
3) Heterogeneity of stem cells leads to differences in therapeutic effects: Within the same stem cell population, there exist biological differences in aspects such as gene expression, protein expression, epigenetic modifications, cell morphology, and metabolic pathways. Although multiple functional subpopulations can be identified through technologies such as single-cell RNA sequencing and the main functional differences of each subpopulation can be analyzed, the technical difficulties in sorting the cells of the required functional subpopulations and the difficulty in customizing special culture media still need to be addressed. Only by overcoming these difficulties can the consistency of treatment be greatly improved and the therapeutic effect be enhanced.
4) Production standardization challenges: Different sources, culture conditions, and cryopreservation techniques can all lead to heterogeneity of MSCs, thereby causing fluctuations in therapeutic effects. In 2024, only two MSC products were approved globally, highlighting the significance of standardized production and quality control. Each type of stem cell MSCs product needs to strictly control the production process and unify standards to reduce possible differences in therapeutic effects.
There is no doubt that regenerative medicine will be one of the important directions for the development of the medical field in the 21st century. Although it is still in its early stage compared with other therapies as a whole, except for a few areas such as the lungs, joints and nervous system where research has taken a long time, there are many gaps in other fields and the market is promising.