
The year 2025 will be a year of "big explosion" for the domestic stem cell industry.
According to incomplete statistics, the number of IND applications for new stem cell drugs in China has exceeded 30 in the first half of this year, showing a significant growth trend compared to the 39 applications for the whole of 2024.
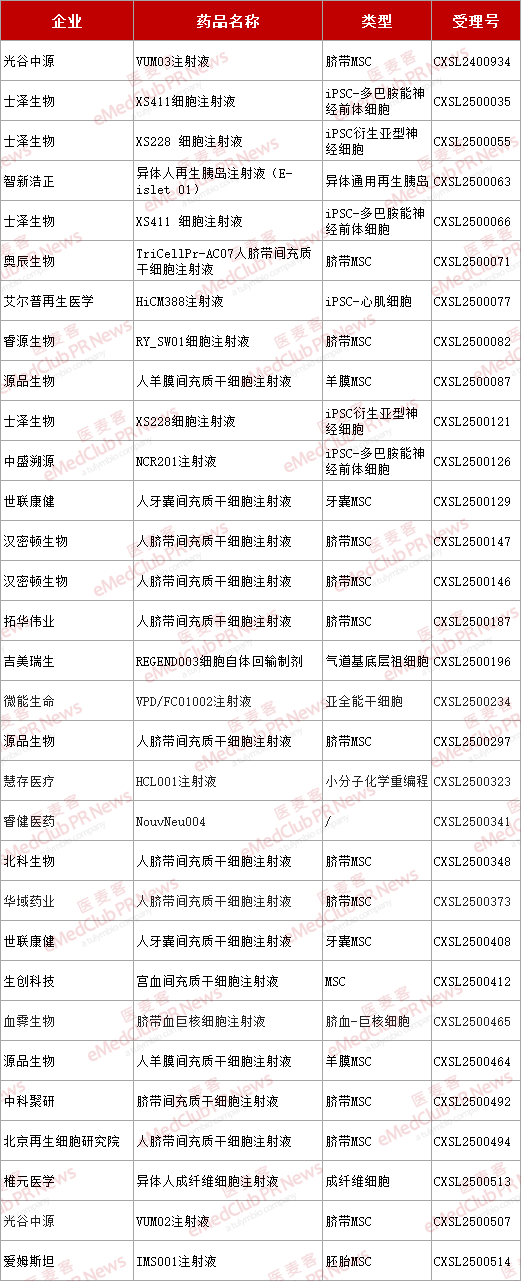
▲ Stem cell projects applying for IND in the first half of 2025
From the perspective of cell type distribution, mesenchymal stromal cells (MSCS) still account for nearly 60% of the IND applications, while pluripotent stem cells represented by ipscs make up less than one-third.
According to the latest report from the Center for Drug Evaluation of the National Medical Products Administration, among the 29 new stem cell inDs added in 2024, 21 are MSC-related, accounting for 72.4%. There is 1 item of multifunctional stem cells, accounting for 3.4%.
Overall, although traditional non-induced MSCS still account for the main force in IND applications, their proportion has decreased compared to previous years. The field of multifunctional stem cells has shown a strong growth momentum, with a significant increase in the number of IND applications, becoming a new focus of the industry.
The core driving force behind this trend shift stems from the increasing maturity of induced pluripotent stem cell (iPSC) technology.
As pluripotent stem cells obtained through gene reprogramming, IPscs not only possess differentiation potential similar to that of embryonic stem cells, but also break through ethical limitations and the challenges of immune rejection. More importantly, unlike traditional stem cells, IPscs have the ability to expand infinitely, which can provide a standardized source of "seed cells" for "off-the-shelf" cell therapy products and also offer a brand-new possibility to break through the long-standing bottlenecks of scale and standardization faced by cell therapy.
In the application field, iPSC technology is rapidly advancing mainly along two major directions: immunotherapy and regenerative medicine. It is particularly worth noting that in the field of regenerative medicine, iPSC-MSC (iMSC) is becoming a research hotspot and shows great application prospects in the fields of anti-inflammation and tissue repair.
iMSC: The next-generation therapy that breaks through the bottleneck of traditional MSC
MSC, with its unique mechanisms such as immune regulation, tissue repair, paracrine effect and immune immunity, has shown broad therapeutic prospects in various disease areas including osteoarthritis and graft-versus-host disease (GVHD), and has become one of the important pillars in the field of regenerative medicine.
At present, the MSCS used for research and treatment mainly originate from tissues such as bone marrow, fat and umbilical cord. However, when traditional sources of MSCS move towards large-scale clinical application and industrialization, they will encounter many challenges such as insufficient consistency, limited large-scale production capacity, and unstable therapeutic effects. These bottlenecks directly lead to low clinical transformation efficiency, high industrial heat and numerous pipelines, but scarce achievements.
With the development of iPSC technology, iMSC has demonstrated potentially irreplaceable advantages in breaking through the above-mentioned bottlenecks.
In fact, iMSC is more similar to MSC in the fetal stage and is the youngest type of MSC. It can be mass-produced while ensuring the stability of cell quantity and quality between batches. However, the number of cells from adult sources is limited, and the quality and efficacy of MSC cells from different donors may be unstable. This might also be one of the fundamental reasons why many products perform poorly in the market after being launched.
Take the first MSC therapy in the United States, Ryoncil, as an example. After more than ten years of efforts and multiple rejections, it was finally approved for marketing by the FDA at the end of 2024. However, as the CMC issue raised in the FDA's CRL, Mesoblast cannot guarantee that the quality of MSCS derived from bone marrow of different donors is consistent, and these problems may all affect the clinical efficacy of MSCS.
The MSCS differentiated from iPSC cells are actually a type of cell, and their characteristics are different from those of MSCS produced through different differentiation pathways. The actual therapeutic effect of the latter and the indications it can effectively target are not necessarily the same.
Furthermore, iPSC can establish master cell banks and working cell banks with unlimited proliferation, fundamentally solving the problems of limited cell sources and inconsistent starting cells between batches. Each batch of IMscs is derived from iPSC clones with the same genetic background, which can significantly enhance the consistency and stability between batches.
Based on the iPSC differentiation production route, the originally complex upstream process of obtaining primary cells can also be replaced by controllable directional differentiation starting from a standardized cell bank, greatly simplifying the process flow. Meanwhile, as the starting cells are standardized cell lines, they are highly suitable for building closed, automated and modular production process platforms. The hollow fiber bioreactor of Termobiest is one of the ideal choices for such platforms. Its perfusion structure design features a high surface area to volume ratio, continuous oxygen supply and the ability to remove metabolic waste, which can create a stable and high-quality microenvironment for cell growth. The successful application of this system in multiple global stem cell projects has demonstrated that it can simultaneously achieve MSC amplification and exosome collection in a closed environment, significantly simplifying the process flow and enhancing batch consistency, providing a replicable path for large-scale production and CMC compliance.
This platform can minimize human intervention, reduce pollution risks and enhance production efficiency and quality stability. Standardized and automated production will eventually bring about a scale effect and significantly reduce production costs. Consistent product quality can also reduce the cost of QC testing, enhance overall commercial feasibility, and is expected to benefit more patients.
The Road ahead: Industrialization and Clinical Transformation
Although iMSC has a promising future, as an emerging technology field, its industrialization and clinical transformation still face production process and regulatory challenges. At the regulatory level, the entire CMC process of iMSC patent drugs needs to be standardized. At the enterprise level, production involves multiple complex links such as reprogramming, database building, and quality control, which require continuous exploration and optimization.
In the future, as iMSC technology gradually matures, iMSC products will accelerate their entry into the clinical transformation and industrialization stage.