
Source: Nature
Cancer cells receive extracellular signal input to obtain a dry-like state, however, how tumor microenvironment (TME) nerve signals guide cancer to establish a hierarchical tumor structure is unclear.
On July 19, 2023, Research paper "trans-activating nucleus-mitochondria coordination to acquire stemness, which reveals that cancer cells use microenvironmental nerve signals to trans-activate nucleus-mitochondrial coordination to acquire stemness. The study conducted pan-cancer transcriptomic screening of 10,852 samples from 33 TCGA cancer types and found that cAMP response element (CRE) transcription factors are polymeric activators of cancer stem. Deconvolution of transcriptome profiles, specification of neuromarkers, and description of norepinephrine kinetics revealed a link between TME nerve signaling and CRE activity in cancer cells.
Specifically, the nerve signal norepinephrine enhances the dryness of proximal cancer cells by activating the cAMP-CRE axis, of which ATF1 is a conserved hub. Under the activation of norepinephrine, ATF1 coordinates nuclear remodeling and mitochondrial regeneration by coordinating trans-activation of nuclear pluripotent factor MYC/NANOG and mitochondrial biogenesis regulatory factor NRF1/TFAM, thereby enhancing cancer dryness. Thus, the single-cell transcriptome confirms the coordinated activation of cancer dry-like nuclear pluripotency with mitochondrial biogenesis. Together, these findings shed light on the fact that cancer cells acquire dryness through a norepinephrine-ATF1-driven nucleo-mitochondrial collaborative program, suggesting that dryness is acquired by hijacking the spatialization of neural signals in the microenvironment.

Cancer stem is clonogenic, self-renewing and multiline differentiation, and is currently a functionally defined plastic cell state. The plasticity of the cancer's dry state suggests that extracellular inputs are required to indicate dryness through complex interactions between intracellular and external factors. Multifunctional transcription factors that control the fate of normal stem cells are central intrinsic regulators of cancer dry like cells (CSCs). Transcription factors such as MYC drive dryness in a variety of cancers, including breast and pancreatic cancer. Extracellular cytokines from the tumor microenvironment (TME), such as TGF-β and IL-6, are sufficient to initiate the dry-like state.
Among the TME factors, nerve signals are increasingly thought to contribute to cancer progression. For example, norepinephrine, gamma-aminobutyric acid, and glutamate have been found to cause an aggressive cancer phenotype, which indicates a dry-like nature of cancer. In fact, TME nerve signals have recently been identified as a signaling hub connecting mental states and cancer progression. However, little is known about how TME-targeted nerve signals combine with intracellular intrinsic dry factors to establish a cancer dry-like state.
Neurotransmitters are sought-after nerve signals involved in cancer development. Among neurotransmitters, norepinephrine, which is induced by adrenergic signaling, plays an important role in cancer progression. Norepinephrine-activated adrenergic receptors regulate the activity of transmembrane adenylate cyclase, which produces 3',5' -cyclic adenosine phosphate (cAMP) to control the cell's response to nerve signals. The transcriptional response to cAMP is primarily dependent on protein kinase A (PKA), which phosphorylates and activates cAMP response elements (CRE) in conjunction with the transcription factor CREB/ATF1 (activating transcription factor 1) to control target gene transcription.
Therefore, CRE binding transcription factors are essential for the cellular response to chronic stress-induced adrenergic neurotransmitters. However, the mechanism of action of adrenergic signal-induced CRE signaling on cancer dryness is unclear. The finding that CDK5-CREB1 enhances glioma stem cells and AMPK-CREB1 enhances glioblastoma stem cells suggests a role for the cAMP response program in dryness. A recent study in metastatic colorectal cancer confirmed that cAMP-CREB1 signaling enhances cancer dryness. However, it is uncertain whether cancer cells acquire dryness through the TME nerve signal-dependent CRE program.
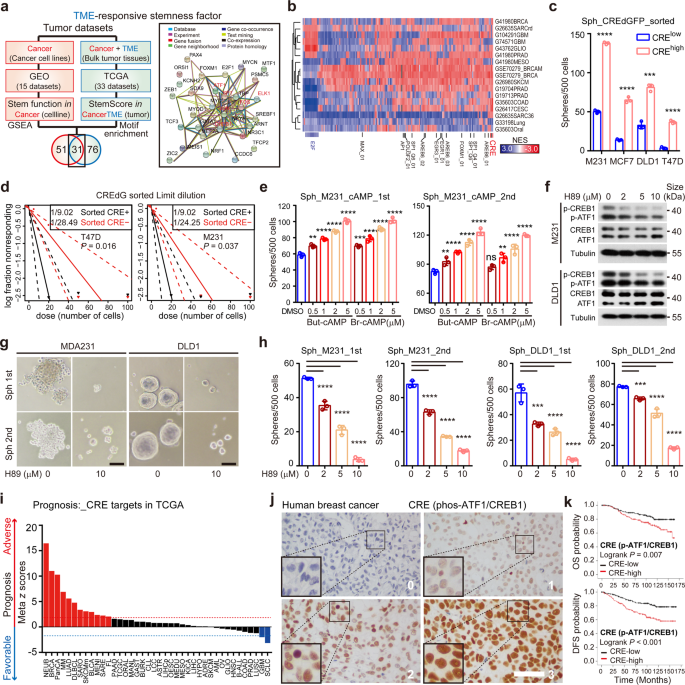 CRE is a pan-cancer TME reactive dry effector
CRE is a pan-cancer TME reactive dry effector
The study characterized pan-cancer TME-reactive dry transcription factors based on dry transcription profiles of 10,852 tumors from 33 TCGA cancer types and 17 pairs of adhesion molecules from 11 cancer types. Among the TME reactive dry factors, CRE factors are conservatively enriched and require dry-like function in multiple cancer types. Deconvolution of tumor cell composition, immunofluorescence, and time-delay fluorescence imaging revealed a link between TME nerve signaling and CRE activity in cancer cells. Specifically, microenvironmental norepinephrine, an adrenergic neurotransmitter activated by chronic stress, enhances the dry activity of proximal cancer cells via the cAMP-ATF1 axis. ATF1 pushes cancer cells to a drylike state by simultaneously coordinating the trans-activation of genes associated with nuclear pluripotency and mitochondrial regeneration, integrating adrenergic signaling and intracellular intrinsic dry factors.