
Source: BioValley
On July 28, 2023, The international academic journal Advanced Science published online a research paper titled "hESC-Derived Epicardial Cells Promote Repair" published by Yang Huangtian's research team at the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, in collaboration with Gao Ling's research team at Dongfang Hospital Affiliated to Tongji University of Infarcted Hearts in Mouse and Swine ". This study found that post-transplantation of human pluripotent stem cells (hPSCs) derived epicardial cells (hEPs) can improve cardiac function and inhibit scar formation after myocardial infarction (MI) in mice and pigs, and found that hEPs can promote paracrine new factors and new mechanisms of infarct myocardial repair by inhibiting interferon IFN-b mediated inflammatory response.
Myocardial infarction leads to irreversible death of cardiomyocytes, and the lost cardiomyocytes are replaced by fiber scars, which leads to maladaptation of heart function and even heart failure, which seriously threatens the health and life of patients. At the same time, myocardial infarction leads to the death of other non-myocardial cells and the destruction of myocardial tissue. However, the excessive inflammatory response after AMI aggravated the myocardial injury. Therefore, it is of great significance to moderately regulate the inflammatory microenvironment after AMI and activate the endogenous cardiac repair mechanism and methods for developing strategies to promote the repair of infarcted myocartis. Epicardial cells are mainly distributed in the visceral layer of the pericardium and are mesothelial cells, which participate in the regulation of coronary artery formation and cardiomyocyte proliferation through epithelial mesenchymal transformation during the development of the heart. Epicardium derived cells isolated from transplanted hearts can promote the repair of infarcted myocardium in mice, but the mechanism is far from clear. Co-transplantation of hEPs with HPSCS-derived cardiomyocytes (hCMs) as supporting cells promotes the maturation and retention of hCMs myofilament structures, but it is unclear whether hEPs can promote the repair of infarcted myocards.
Using mouse and pig myocardial infarction models and combined with a variety of research methods, the researchers found that hEPs transplantation in the acute stage of myocardial infarction promoted the recovery of cardiac function and inhibited scar formation in mice, and was accompanied by reduced mononuclear/macrophage infiltration, increased repair macrophages, and promoted the survival of cardiomyocytes and the regeneration of blood vessels and lymphatics. Mechanism analysis showed that hEPs transplantation significantly inhibited the inflammatory response of infarcted myocartis, especially down-regulated type I interferon (IFN-I) signal. IFN-I receptor agonist RO8191 weakened the HEPS-mediated inhibition of inflammatory response and promoted the polarization of repair macrophages and myocardial repair. Mass spectrometry screening and multi-party verification showed that hEPs secreted endagglutinin (ITLN1), which can bind to IFN-β, mimics the effect of hEPs on inhibiting IFN-I-mediated inflammatory response and promoting the polarization of repair macrophages. Transplantation of hEPs with ITLN1 knockdown can cancel the effects of hEPs on IFN-I signaling, inflammation regulation and myocardial repair in the infarcted heart. Further, in a more clinical model of porcine myocardial infarction, it was demonstrated that hEPs transplantation inhibited inflammation and promoted the repair of porcine infarcted myocardities, and no organ toxicity was observed.
In conclusion, hEPs transplantation promotes myocardial repair in mice and pigs with infarction. One of the main mechanisms is the interaction between ITLN1 secreted by hEPs and IFN-β, which further inhibits macrophage inflammation mediated by IFN-I pathway and promotes the polarization of repair macrophages. This study revealed the important role of hEPs and secreted protein factors in regulating inflammatory response by inhibiting IFN-I pathway, thus promoting myocardial repair, and found a new mode of regulating IFN-β and a new mechanism of regulating inflammatory response. It is suggested that hEPs may be a new cell source for myocardial repair after myocardial infarction by precisely regulating the inflammatory response.
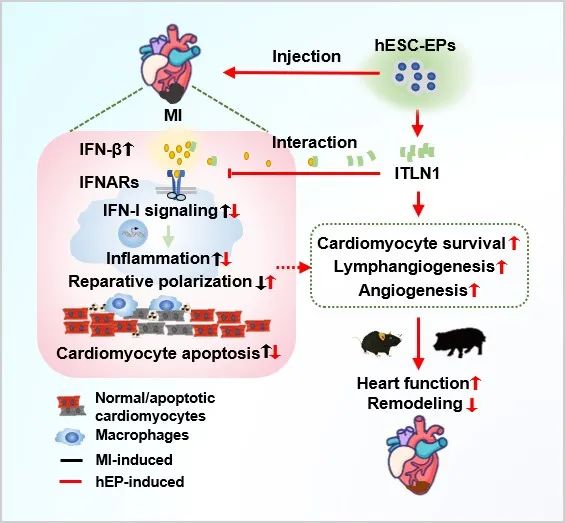
The model of HPSCS-derived epicardial cells regulating inflammation and promoting the repair of infarcted myocardium by secreting endogenous agglutinin
The original link: https://doi.org/10.1002/advs.202300470