
Source: biological world
Pancreatic cancer (Pancreatic Cancer) is a malignant tumor of the digestive tract, which is very malignant and difficult to diagnose and treat. Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 95% of all pancreatic cancer. The 5-year survival rate of PDAC is only 11%, making it the most malignant solid tumor.
Chemotherapy can significantly prolong the survival time of PDAC patients, but due to the complex and unclear drug resistance mechanism, the response rate of PDAC patients to chemotherapy is still very low. Typing based on genome and transcriptome characteristics does not provide guidance for chemosensitivity or clinical treatment of PDAC. At present, PDAC is still treated as a single type of tumor. Tumor metabolic reprogramming is considered as a new treatment resistance mechanism. However, the abundant stromal cells in PDAC make it difficult to capture tumor-specific metabolic information.
As a new research model, tumor-like organs derived from patients have been proved to be superior to traditional cell lines in reproducing the characteristics of primary tumors.The use of organ-like model to deeply explore the metabonomic characteristics of pancreatic cancer and integrate and analyze multi-group data to reveal the metabolic differences and potential drug targets of pancreatic cancer has important scientific value and clinical application prospects.
August 18, 2023, Jin Gang Research Group of the first affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Gao Dong Research Group, Chen Luonan Research Group, Yang Weiwei Research Group and Yin Huiyong Research Group of Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences. A research paper entitled Metabolic classification suggests GLUT1/ALDOB/G6PD axis as a therapeutic target in chemotherapy-resistant pancreatic cancer was published in Cell Reports Medicine, a sub-journal of Cell.
This study systematically revealed the metabonomic characteristics of pancreatic ductal adenocarcinoma (PDAC), and proposed the chemosensitivity mechanism and potential therapeutic targets of different metabolic subtypes by integrating metabolic group, whole genome, transcriptome and drug sensitivity data.

The research team systematically collected metabonomics, whole genomics, transcriptome, chromatin openness and drug sensitivity information based on 28 pancreatic cancer-like organs. Two metabolic subtypes of PDAC, enriched glucose metabolism (Glucomet-PDAC) and lipid metabolism (Lipomet-PDAC), were identified by metabonomics. Compared with Lipomet-PDAC, Glucomet-PDAC is more resistant to chemotherapy, and patients with Glucomet-PDAC characteristics have a poorer prognosis.
Further integration of multi-group studies found that the GLUT1/ALDOB/G6PD metabolic axis plays an important role in the metabolic reprogramming of Glucomet-PDAC. First of all, in Glucomet-PDAC, Glut1 is highly expressed, transporting more glucose into tumor cells. Secondly, the decreased expression of ALDOB released the activity of G6PD and increased the metabolic flux of oxidized pentose phosphate pathway.
With the increase of metabolic flux of oxidized pentose phosphate pathway, more raw materials involved in DNA synthesis were synthesized in Glucomet-PDAC, which induced their resistance to chemotherapeutic drugs. Knocking down GLUT1, overexpressing ALDOB or knocking down G6PD in Glucomet-PDAC can increase the sensitivity of cells to chemotherapeutic drugs. Similarly, inhibitors of GLUT1 and G6PD can increase the sensitivity of PDAC to chemotherapeutic drugs in vitro and in vivo.
Finally, through virtual screening and in vitro and in vivo verification, it is found that two clinical inhibitors of Aurora, MLN8054 and Alisertib, can effectively inhibit the activity of G6PD and increase the sensitivity of chemotherapeutic drugs. This study revealed the potential metabolic heterogeneity associated with PDAC chemosensitivity and developed a promising pharmacological strategy for chemotherapy-resistant glucomet-PDAC patients through a combination of chemotherapy and GLUT1/ALDOB/G6PD axis inhibitors.
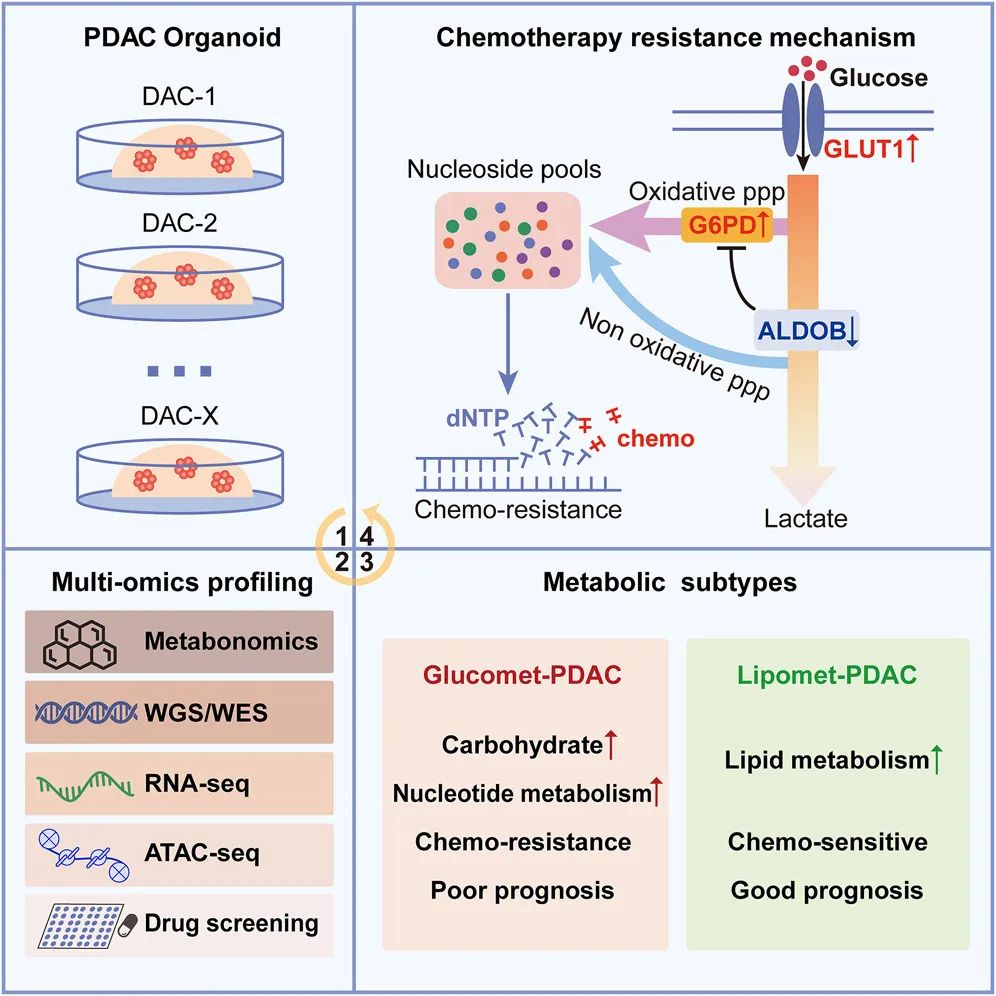
Metabolic typing of Pancreatic Cancer and Drug Resistance pattern induced by reprogramming
Researcher Gao Dong, researcher Chen Luonan, researcher Yang Weiwei, researcher Yin Huiyong, Institute of Nutrition and Health, Chinese Academy of Sciences, and Professor Jin Gang, Department of Hepatobiliary, Pancreatic and splenic surgery of Shanghai Changhai Hospital are the co-authors of this paper.
Postdoctoral students Li Yunguang, Tang Shijie, Wu Xueyuan, Zhang Han, Department of Hepatobiliary, Pancreatic and splenic surgery of Shanghai Changhai Hospital, and Lu Jingwen, Ph.D., Institute of Nutrition and Health are co-first authors of this paper.
The research work is supported by the National key Research and Development Program, the National Natural Science Foundation, the Strategic pilot Science and Technology Project of the Chinese Academy of Sciences, and the Shanghai Science and Technology Commission. This research work is supported by the molecular cell center of excellence cell analysis technology platform, chemical biology technology platform and animal experiment technology platform.
Paper link: https://doi.org/10.1016/j.xcrm.2023.101162