
Source:BioArtMED
Alzheimer's disease (Alzheimer's disease AD) is still a major challenge facing mankind at present. The pathogenesis of the disease is complex and controversial.The family pathogenesis of AD is caused by the hydrolysis and processing mutation of Alzheimer's disease precursor protein (Alzheimer precursor protein APP), which leads to the excessive production of APP cleavage product β-amyloid protein, which is the main component of senile plaque and is the diagnostic standard of Alzheimer's disease.
According to the genome-wide association study of sporadic delayed Alzheimer's disease, it was found that there were many mutations in the microglia genome of patients, including TREM2R47H mutation, which is a high genetic risk mutation in patients with AD. TREM2 mutations in microglia can lead to cell dysfunction and lead to early-onset neurodegenerative syndrome. Restoring and enhancing the function of microglia is a very potential therapeutic strategy.
On August 3, 2023, the Marius Wernig team from Stanford University published an article entitled A cell therapy approach to restore microglial Trem2 function in a mouse model of Alzheimer's disease on Cell Stem Cell. In this paper, it is found that microglia substitution can effectively restore the function of microglia in the mouse model of Alzheimer's disease.
First, the authors used an AD mouse model to study whether circulating myeloid cells (circulation-derived myeloid cells CDMCs) can replace resident microglia in the brain.The mouse model 5xFAD was hybridized with GFP mice. The bone marrow cells of the hybridized mice were used as donor cells, and microglia were replaced by bone marrow transplantation.CSF1R inhibitors were treated briefly after bone marrow transplantation in three-week-old mice. Flow cytometry and histological analysis showed that the effective chimerism rate of transplanted cells in peripheral blood and brain of mice was more than 90% and lasted for at least 5 months. The chimerism rate in the control group that received only transplantation was only 20%. Histological analysis showed that GFP+ cells were widely and uniformly incorporated. The authors examined the function of the transplanted cells and found that the pathological response of CDMC to Alzheimer's disease was similar to that of microglia in a mouse model.
Next, the authors transplanted bone marrow cells from Trem2+/+; 5xFAD; Ubc-GFP mice into Trem2-/-; 5xFAD mice. It was found that the activity of microglia in Trem2-/-; 5xFAD was restored after microglia replacement. The number of myeloid cells in Iba-1+ recovered but not completely.Functional analysis showed that transplanted Trem2+/+CDMC could reduce amyloid deposition, reduce LAMP1+ synaptic dystrophy and restore antibody plaque phagocytosis.Transcriptome analysis showed that after microglia were replaced, the expression of neurodegenerative disease related transcriptional group was restored. The expression of the related transcriptional group depended on Trem2 and endowed microglia with neuroprotective function. Syk is not only an important signal pathway downstream of Trem2 receptors, but also an important indicator of Trem2 activation. The authors found that after the microglia were replaced, the genes targeting Syk signals were restored, and the Syk-mediated signal receptors, namely surface receptors Clec7 and CD11c, were also restored.
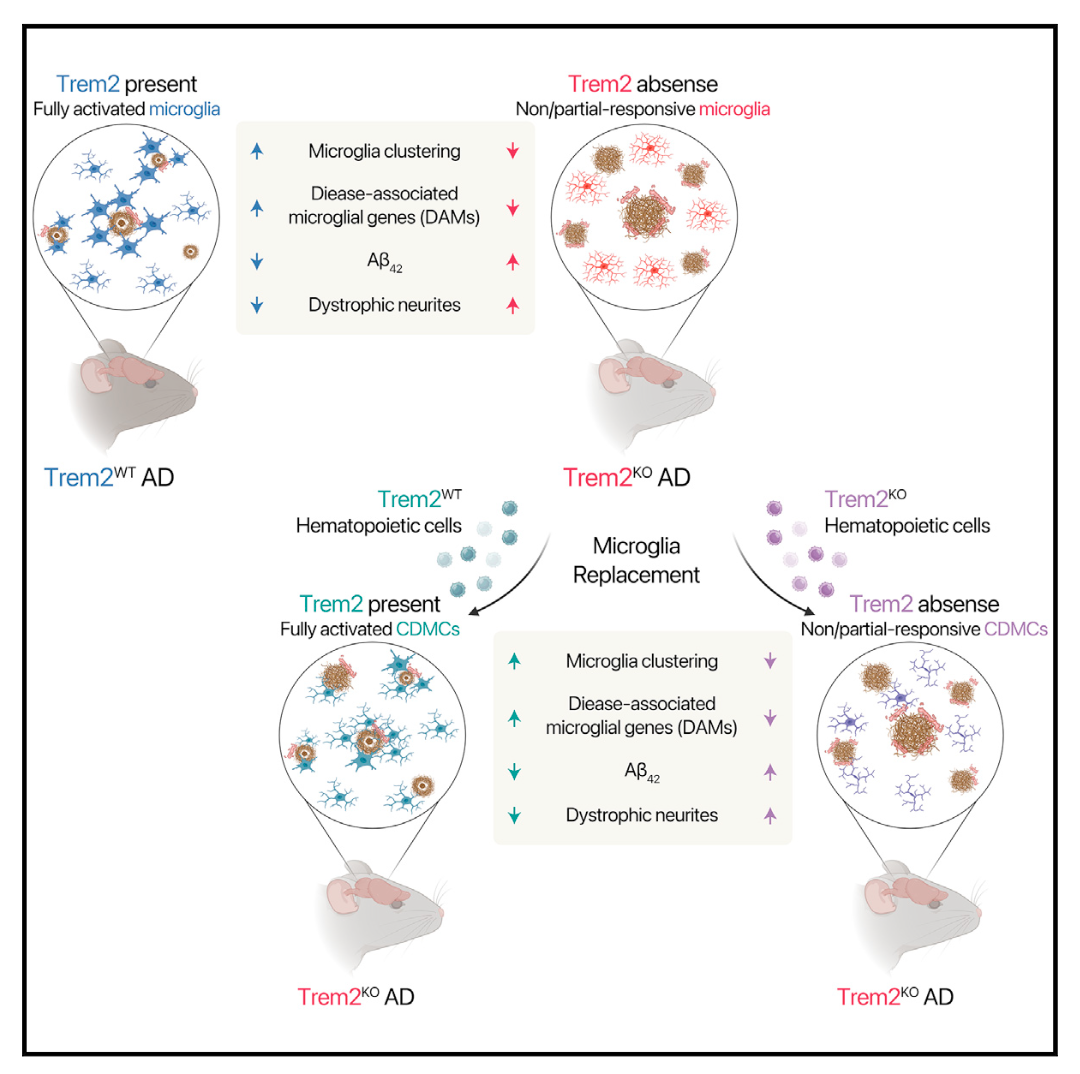
This study shows that systemic hematopoietic stem cell transplantation can replace mouse microglia and restore the function of microglia in the Alzheimer's disease model of Trem2 mutant mice. It also shows that efficient microglia replacement is a feasible choice for Alzheimer's disease in the future. At present, hematopoietic stem cell transplantation requires toxic pretreatment of bone marrow in advance, which hinders its application in disease treatment. Non-toxic pretreatment is a top priority in the future.
Original link: https://doi.org/10.1016/j.stem.2023.07.006