
Source: BioArtMED
Zika virus can infect fetuses through vertical transmission and cause severe neurological diseases such as microcephaly in fetuses, posing a serious threat to maternal and fetal health. The placenta is an important temporary organ that connects the mother and fetus during pregnancy, not only responsible for the exchange of fetal nutrients and gases, but also acts as a barrier to protect the fetus from foreign pathogens. Placental trophoblast cells are the main cell types that perform placental functions, including cytotrophoblast cells (CTB) and syncytiotrophoblast cells (STB) and extrvillus trophoblast cells (EVT), which are derived from CTB. However, the effects of Zika virus infection on the development and function of placental trophoblast cells and the mechanisms of infection remain unclear due to the lack of suitable functional study models in vitro.
On September 8, 2023, Chengfeng Qin, a researcher at the Academy of Military Medicine, and Hongmei Wang, a researcher at the Institute of Zoology, Chinese Academy of Sciences, collaborated in a paper entitled Zika virustargets human trophoblast stem cells and prevents in Nature Communications syncytialization in placental trophoblast organoids. A Zika virus infection model was established using 3D placental trophoblast organoids derived from human placental trophoblast stem cells (hTSCs), which revealed that undifferentiated hTSCs were the main target cells for Zika virus to infect the placenta, clarified the molecular mechanism of placental dysplasia caused by Zika virus through inhibition of syncytization, and provided a new idea for the study of etiology-maternal-fetal interface.
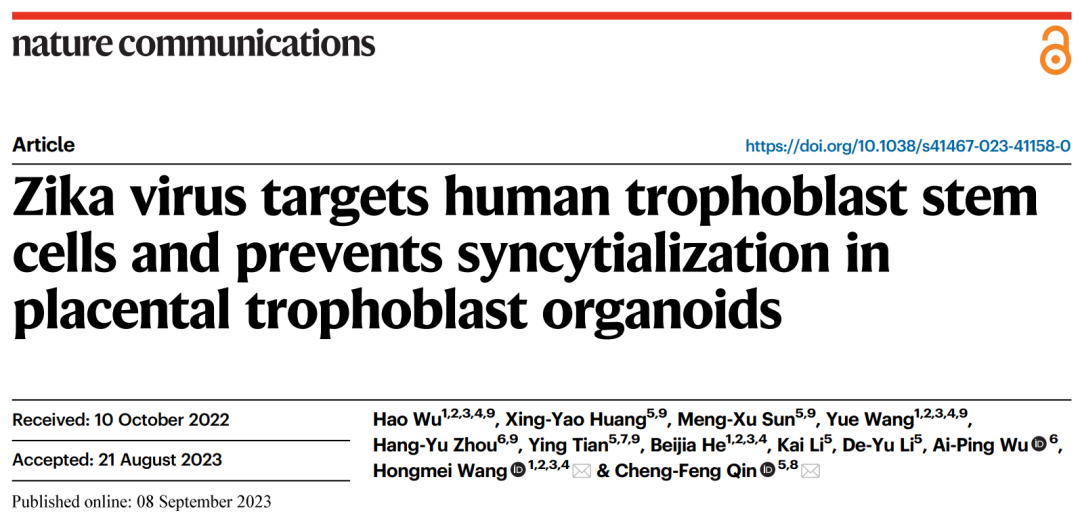
First, in order to clarify the characteristics of Zika virus infection on human placental trophoblast cells, the team established a Zika virus infection model using hTSCs differentiated trophoblast cells, demonstrating that hTSCs are the main target cells of Zika virus, and their susceptibility to Zika virus decreases with hTSCs differentiation. The researchers further used hTSCs to build a 3D placental trophoblast organoid that mimics the gene expression, hormone secretion, and differentiation potential of primary trophoblast cells, and established a model of the trophoblast organoid infected by Zika virus. It was found that Zika virus can effectively infect trophoblast organoids and directly destroy the structure of mature trophoblast organoids. With the increase of infection dose, Zika virus gradually infected from the peripheral CTB to the inner layer STB. To investigate the long-term effects of Zika virus on the development of trophoblast organoids, we established infection in the early stage of trophoblast organoid development (Day1) and observed STB in Day8. The results showed that the development of STB in the trophoblast organoids was significantly limited or scattered. Immunofluorescence staining (IF) and fusion efficiency statistics demonstrated that Zika virus infection significantly inhibited trophoblast syncytization.
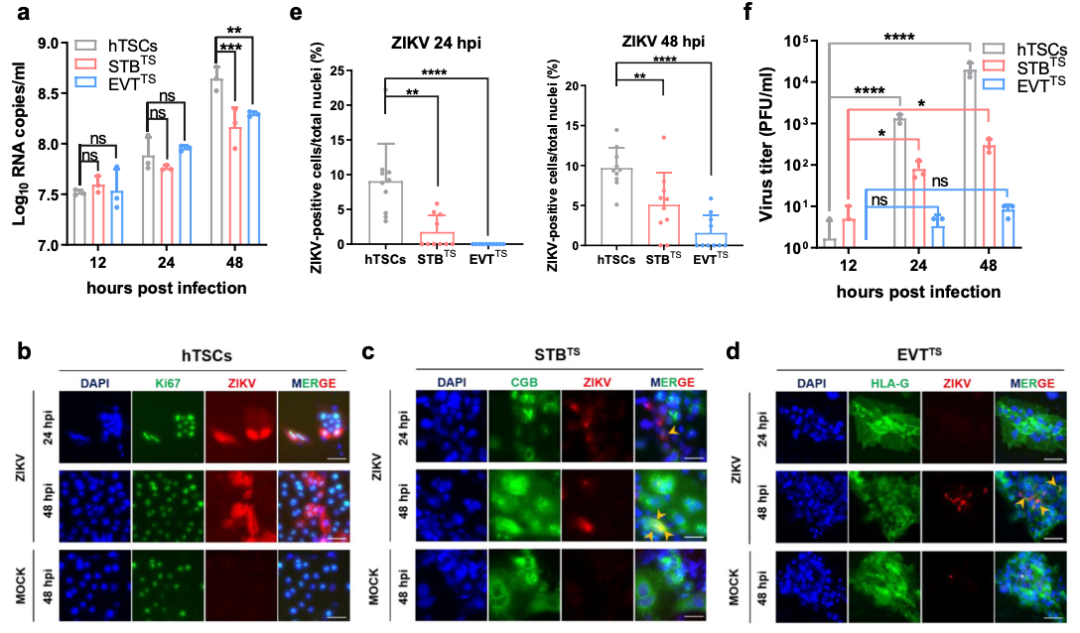
hTSCs, STBTS, and EVTTS have different susceptibility to Zika virus (ZIKV) infection
To further elucidate the effects of Zika virus infection on the development of trophoblast organoids, we compared the transcriptome changes of various trophoblast cells in the trophoblast organoids before and after Zika virus infection at the single-cell level. Firstly, the cell composition in trophoblast organoids was analyzed, which mainly included hTSC, CTB, CTB_Fusion and STB. Trajectory analysis proved that cell development in trophoblast organoids could simulate the process of trophoblast cell syncytization. Further, the authors analyzed the effects of Zika virus infection on trophoblast organoids. Using MX1 as a marker to distinguish infected cells, the analysis results showed that the proportion of MX1 positive cells gradually decreased with CTB syncytization, indicating that trophoblast cells were less susceptible to Zika virus with differentiation. This is very similar to what is observed in neural precursor cells.
The background immune status of cells was correlated with their susceptibility to viruses, and analysis showed that hTSCs lacked background expression of IFN and ISG known to have important antiviral effects in organoids, which partly explained the high susceptibility of hTSCs to Zika virus. After Zika virus infection, some classical antiviral IFN and ISG in hTSCs are activated, especially IFITM1/3, which is known to have extensive antiviral effects, is activated in hTSCs after infection. Previous studies have reported that high expression of IFITM in trophoblast cells may inhibit the syncytization of trophoblast cells. This suggests that syncytification of hTSCs may be limited by Zika virus infection. Overdifferentially expressed genes and pathway enrichment analysis showed that Zika virus infection may inhibit the stem of hTSCs and the proliferation of CTB, and may affect the hormone secretion function of trophoblast cells. The changes of polypeptide hormone coding genes in STB after infection show the characteristics of preeclampsia.
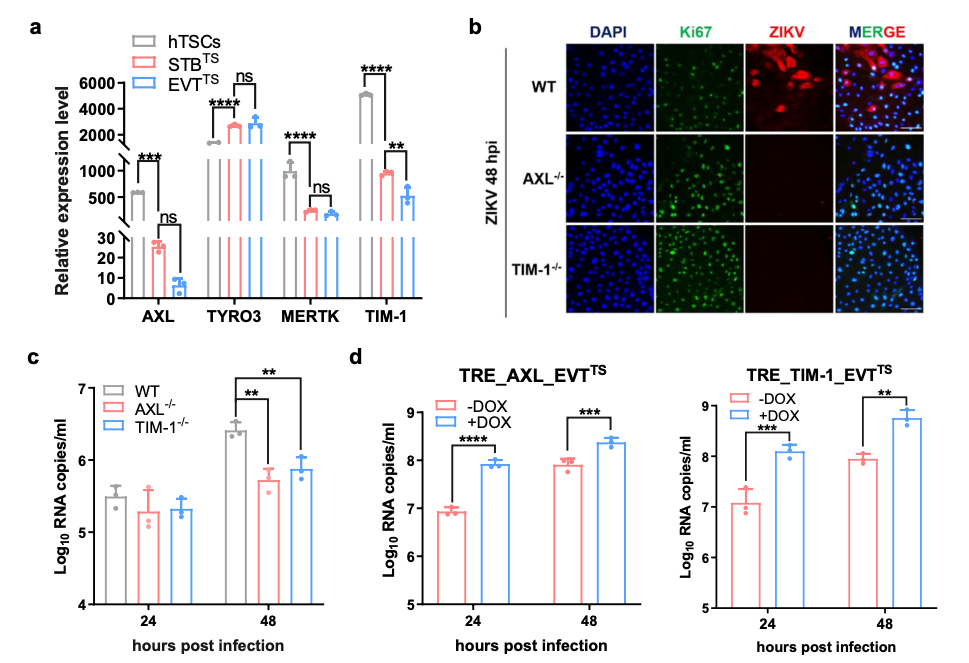
The expression of AXL and TIM-1 promoted ZIKV infection in hTSCs
In summary, this study used HTSCS-derived placental trophoblast cells and 3D placental trophoblast organoid models to truly reproduce the key nodes and processes of Zika virus infection of placenta, and revealed the molecular mechanism leading to developmental abnormalities, providing a new perspective for a deep understanding of the pathogenic mechanism of Zika virus and a new target for the development of specific prevention and treatment drugs.
Qin Chengfeng, a researcher from the Academy of Military Medicine, and Wang Hongmei, a researcher from the Institute of Zoology of the Chinese Academy of Sciences, are co-corresponding authors of the paper. Wu Hao, postdoctoral fellow, Wang Yue, doctoral candidate, Institute of Zoology, Chinese Academy of Medical Sciences; Huang Xingyao, assistant researcher, Sun Mengxu, postdoctoral researcher, Tian Ying, master candidate, Academy of Military Medical Sciences; and Zhou Hangyu, Associate researcher, Institute of Systems Medicine, Chinese Academy of Medical Sciences are co-first authors of this paper. Wu Aiping, a researcher from the Institute of Systems Medicine, Chinese Academy of Medical Sciences, made important contributions to this study.