
Source: eMedClub News
On October 5, 2023, Neurona Therapeutics announced a paper in Cell Stem Cell reporting advances in a novel regenerative cell therapy strategy for drug-resistant focal epilepsy that demonstrated good robustness and reproducibility of NRTX-1001. NRTX-1001 is a highly purified cortical MGE (medial ganglion eminence) GABA-inhibitory interneuron cell therapy candidate derived from human stem cell lines.
Preclinical data show that transplanting NRTX-1001 cells into animal models with chronic medial temporal epilepsy (MTLE) significantly inhibits focal seizures in animals, reduces the neuropathological phenotype of epilepsy, and improves animal survival. These preclinical findings support the ongoing open-label first-in-human Phase I/II trial of NRTX-1001 (NCT05135091) in adult patients with drug-resistant MTLE, which has shown positive early-stage clinical data.
According to the Centers for Disease Control and Prevention, about 3.4 million Americans have epilepsy, and 25 to 35 percent of them continue to have seizures despite being treated with approved medications, indicating a huge unmet medical need. MTLE, a common type of focal epilepsy in adults, primarily affects the internal structure of the temporal lobe, and seizures usually begin in a structure known as the hippocampus. One-third of patients are resistant to anti-epileptic drugs and can only choose sub-optimal treatment options, such as brain tissue destruction surgery, through surgical removal or laser ablation of the damaged temporal lobe is an option; However, current surgical options are not effective in all patients, are tissue destructive, can produce significant adverse effects, including neurocognitive impairment, and are rarely used for MTLE.
Neurona Therapeutics is a clinical-stage biotherapeutics company that has raised $200 million since its founding in 2008. In June 2021, it received a $41.5 million funding round co-led by UCB Ventures and The Column Group, followed by an $8 million grant from the California Institute for Regenerative Medicine in May 2022. In February, the company said it was laying off 18 of its 68 employees, or 25 percent, because of funding problems.
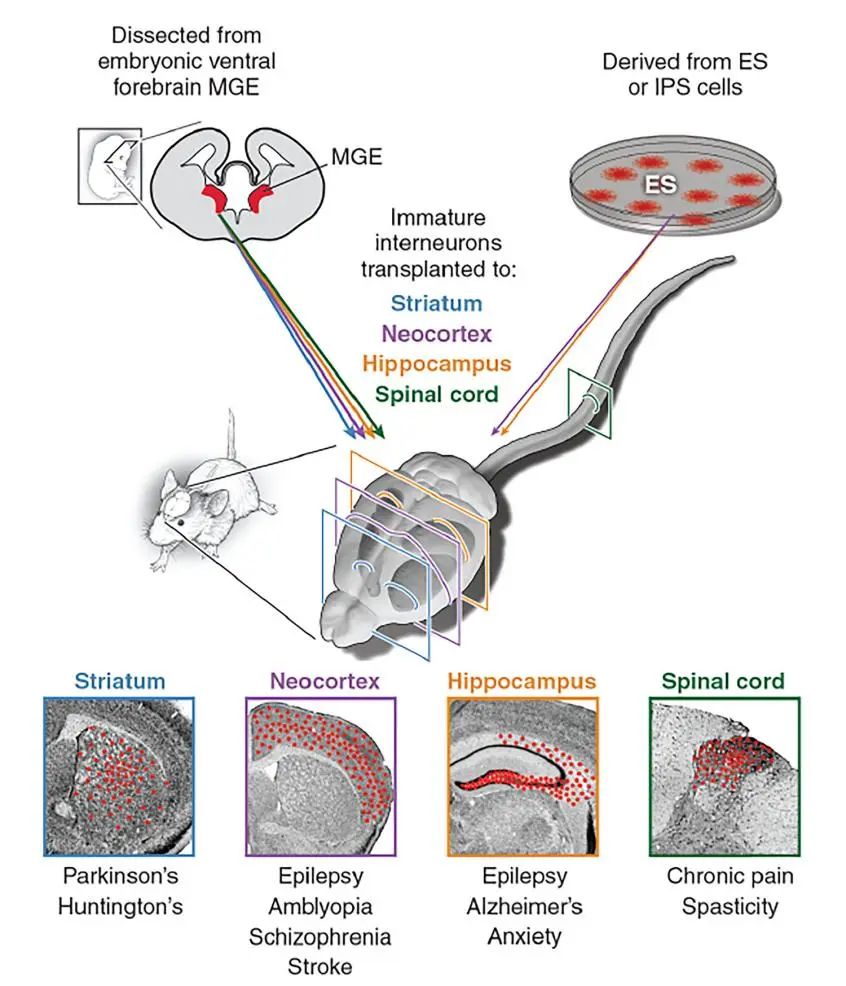
Stem cell neurotherapy model diagram
Neurona is developing off-the-shelf allogeneic nerve cell therapy candidates aimed at long-term recovery of neural network dysfunction in a variety of neurological disorders. In addition to its use in focal epilepsy, Neurona is also developing NRTX-1001 for the treatment of Alzheimer's disease. The company also laid out myelin glial cells, indications have not been announced, in the preclinical stage; And a cellular pipeline for gene editing, which is in the early stages of development.
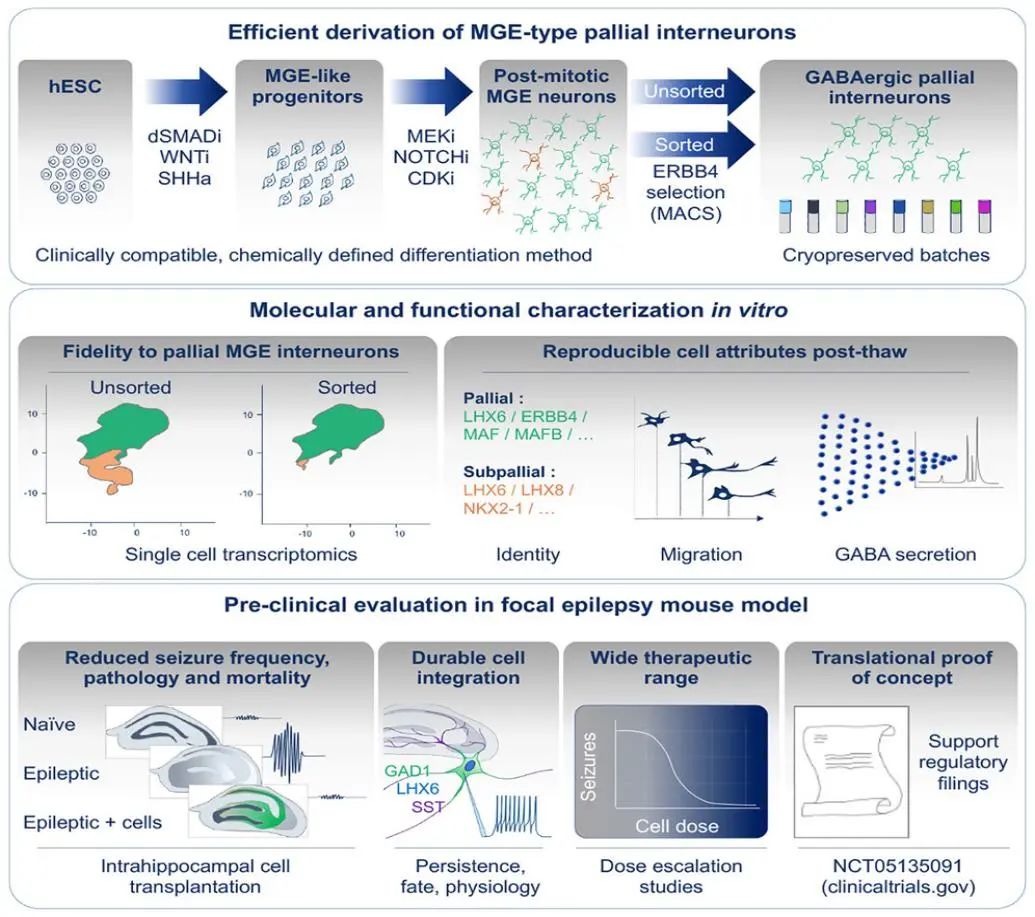
Differentiation, molecular/functional characteristics of MGE cortical interneurons in vitro, and preclinical validation of mouse models
NRTX-1001 is derived from human pluripotent stem cells and was manufactured using a GMP-compliant proprietary manufacturing process. As described in the paper, the cells were evaluated for extensive molecular and functional characterization, including single-cell RNA sequencing, in vitro migration, and GABA secretion analysis. After transplanting NRTX-1001 into the hippocampal site of an animal model of chronic MTLE, the researchers analyzed cell migration and persistence of human interneurons, graft composition, synaptic connectivity, and dose-dependent reduction in seizure activity. In preclinical models, NRTX-1001 had sustained and stable inhibition of seizures in the middle temporal lobe after a single dose of intra hippocampal administration, with seizures no longer occurring in most animals in the treatment group. During the 8.5-month study, transplanted human interneurons showed local dispersion, functional integration, and this phenomenon persisted in the sclerotic hippocampus. Hippocampal dentate granule cell diffusion, a pathological marker of MTLE, was significantly reduced, and the survival rate of epilepsy models increased. The inhibition of seizure activity and the reduction of the neuropathologic phenotype in preclinical animal models were cell dose-dependent, indicating a potentially broad therapeutic dose range of NRTX-1001. After cell transplantation, no abnormal tissue or behavioral abnormalities were found in the animal models at any dose.
The first two subjects to receive NRTX-1001 in the ongoing clinical trial of the drug-resistant MTLE Phase I/II trial in adults both had a significant history of monthly seizure activity that was not controlled by antiepileptic drugs when they entered the study. The first subject had a seven-year history of seizures and had an average of 32 seizures in the six months prior to taking NRTX-1001. The second subject had a nine-year history of seizures and had an average of 14 seizures per month in the six months prior to treatment. Two patients still reported a >95% reduction in the number of total seizures one year after receiving NRTX-1001. According to the treatment protocol, neurocognitive testing began six months after treatment in both patients, and specific neurocognitive scores increased from baseline, highlighting the therapeutic potential of NRTX-1001. So far, no serious or severe side effects of NRTX-1001 have been found.
Cory R. Nicholas, PhD, CEO of the company, commented: "Physiologically, MGE GABA-inhibitory interneurons in the cerebral cortex represent the most relevant cell population for epilepsy cell replacement therapy, however, until now, no suitable, reproducible, scalable cells have been available clinically. I would like to congratulate the entire Neurona team and our collaborators for their commitment to advancing this novel cell therapy strategy that has the potential to offer tissue recovery options to patients with drug-resistant focal epilepsy."
Reference materials:
1.https://doi.org/10.1016/j.stem.2023.08.013
2.https://www.neuronatherapeutics.com
3.https://www.biospace.com/article/releases/neurona-therapeutics-announces-publication-in-cell-stem-cell-reporting-the-development-of-investigational-novel-regenerative-cell-therapy-strategy-for-drug-resistant-focal-epilepsy/