
Source: BioArtMED
The human inner ear is one of the most sophisticated organs in nature, characterized by the spiral cochlea, which consists of an orderly series of auditory sensory cells, as well as a complex vestibular organ. The complex morphology of the inner ear is composed of a series of intricate signal events during fetal development, which has high coordination and high fidelity. Currently, nearly 10% of adults worldwide have moderate to severe hearing loss, but the incidence of hearing loss at birth is less than 0.2%. This means that the vast majority of neuropathic hearing loss is caused by dysfunction of the original inner ear cells in adult development. With the advent of music devices such as headphones, more adults are developing more severe moderate to severe hearing loss.
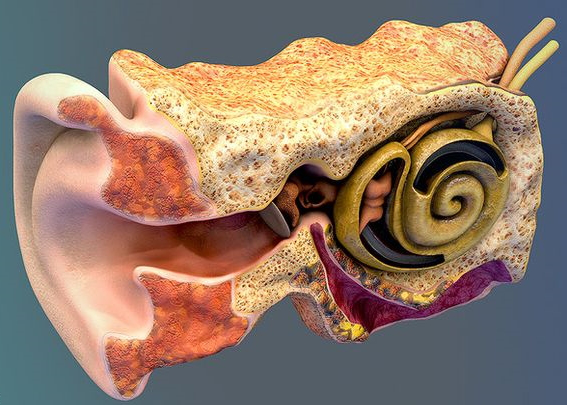
Ear Anatomy, source: Unity asset store
Hearing loss is a global public health problem, with 460 million people worldwide affected by hearing loss, more than 90% of whom are in low - and middle-income countries, according to the WHO. More than 30% of people aged 65 are affected by hearing loss. In addition, more than 1 million newborns are born each year with hearing impairment, and more than 140 million adolescents (ages 12-35) are affected by hearing impairment due to exposure to high volume music and entertainment due to prolonged use of headphones. These data show that hearing loss has become a common public health problem worldwide and requires action to improve it. In recent years, with the deepening of research, stem cells have shown good prospects in the research and treatment of many diseases. Similarly, many new advances have been made in the field of auditory therapy.
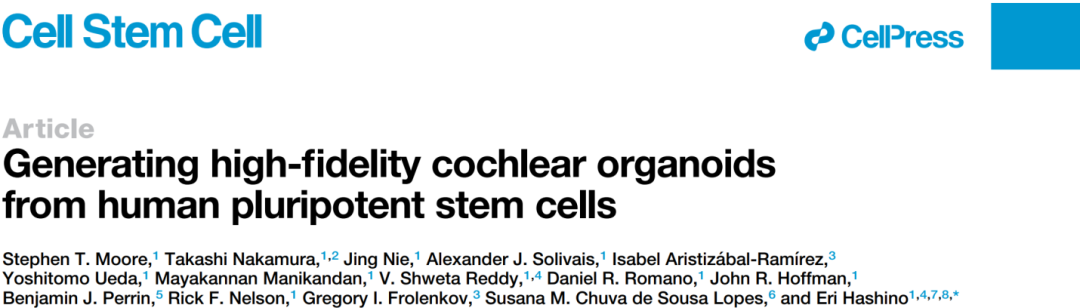
To recreate the complex process of inner ear development in vitro, on July 6, 2023, The Eri Hashino research Group at Indiana University published the title "Generating high-fidelity cochlear organoids from human pluripotent stem" in Cell Stem Cell High fidelity cochlear organoids were established from human induced pluripotent stem cells.
Mechanosensitive hair cells in the cochlea are responsible for hearing, but are vulnerable to genetic mutations and environmental damage. Due to the lack of human cochlear tissue research samples, it is very difficult to study human cochlear tissue and the hair cells in it. The emergence of organoids provides an important platform for the study of cochlear tissue.
To monitor the efficiency of human induced pluripotent stem cell-derived cochlear progenitor cells and hair cells in vitro culture, the authors used CRISPR/Cas9 gene-editing techniques to construct a two-color reporter factor for PAX2-2A-nGFP/POU4F3-2A-ntdTomato. PAX2 is an early marker of cochlear progenitor cells, while POU4F3 is a highly specific marker of differentiated hair cells that can be used to determine the efficiency of cochlear organoid culture (Figure 1).
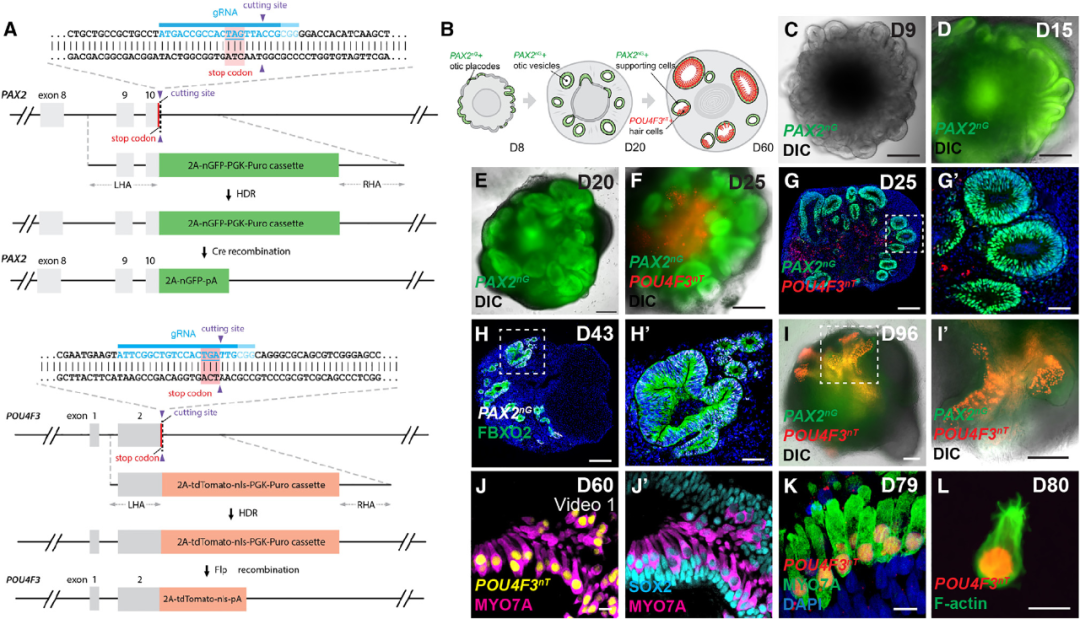
Figure 1 Cochlear organoids expressing PAX2-2A-nGFP/POU4F3-2A-ntdTomato two-color reporter factor
The organoid platform established by the research team relies on the self-induced differentiation of human induced pluripotent stem cells to a certain extent, which is similar to the experimental scheme of inner ear organoids reported previously [3-6]. In order to further optimize the culture system for the sustained production of hair cells and vestibular phenotypes, the authors analyzed key factors in cochlear tissue development. Sonic Hedgehog (SHH) and WNT signaling pathways play key roles in the development of cochlear structures. Therefore, the team sequentially added PUR, a small SHH agonist, and IWP2, a WNT inhibitor, to the induction regimen, and found that it promoted ventral lateralization of inner ear organoids (Figure 2).
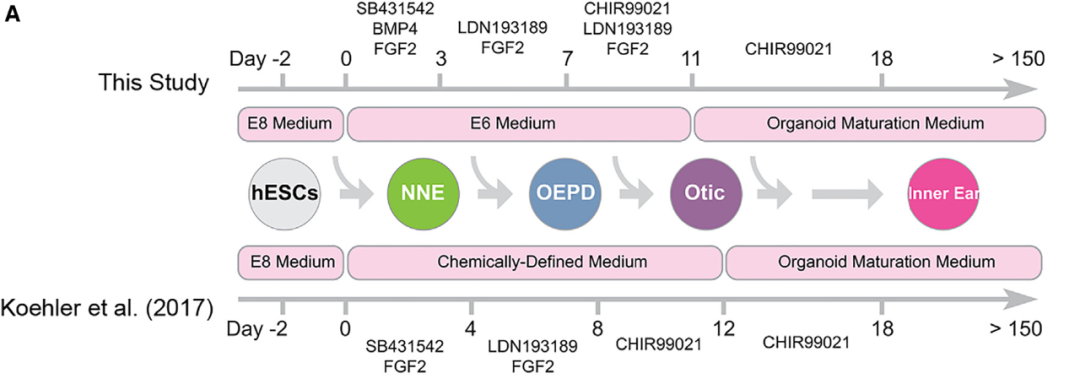
Figure 2 Optimization of cochlear organoids experimental scheme
The research team then performed single-cell RNA sequencing on the inner ear organoids, collecting a total of 37,073 cells to analyze the cochlear cell markers and determine the effectiveness of the induction culture protocol. Treatment with PUR+IWP2 has been found to promote ventral lateralization of ear progenitor cells in human inner ear organoids. In addition, through immunostaining of the markers, the team found that the hair cell markers were expressed in ventrally lateralized inner ear organoids. The results of scanning electron microscopy also showed that the established cochlear organoids showed the structural characteristics of hair cells, and electrophysiological studies also found that the organoids expressed the external hair cell marker PRESTIN and had the characteristic voltage-gated current characteristic of cochlear external hair cells.
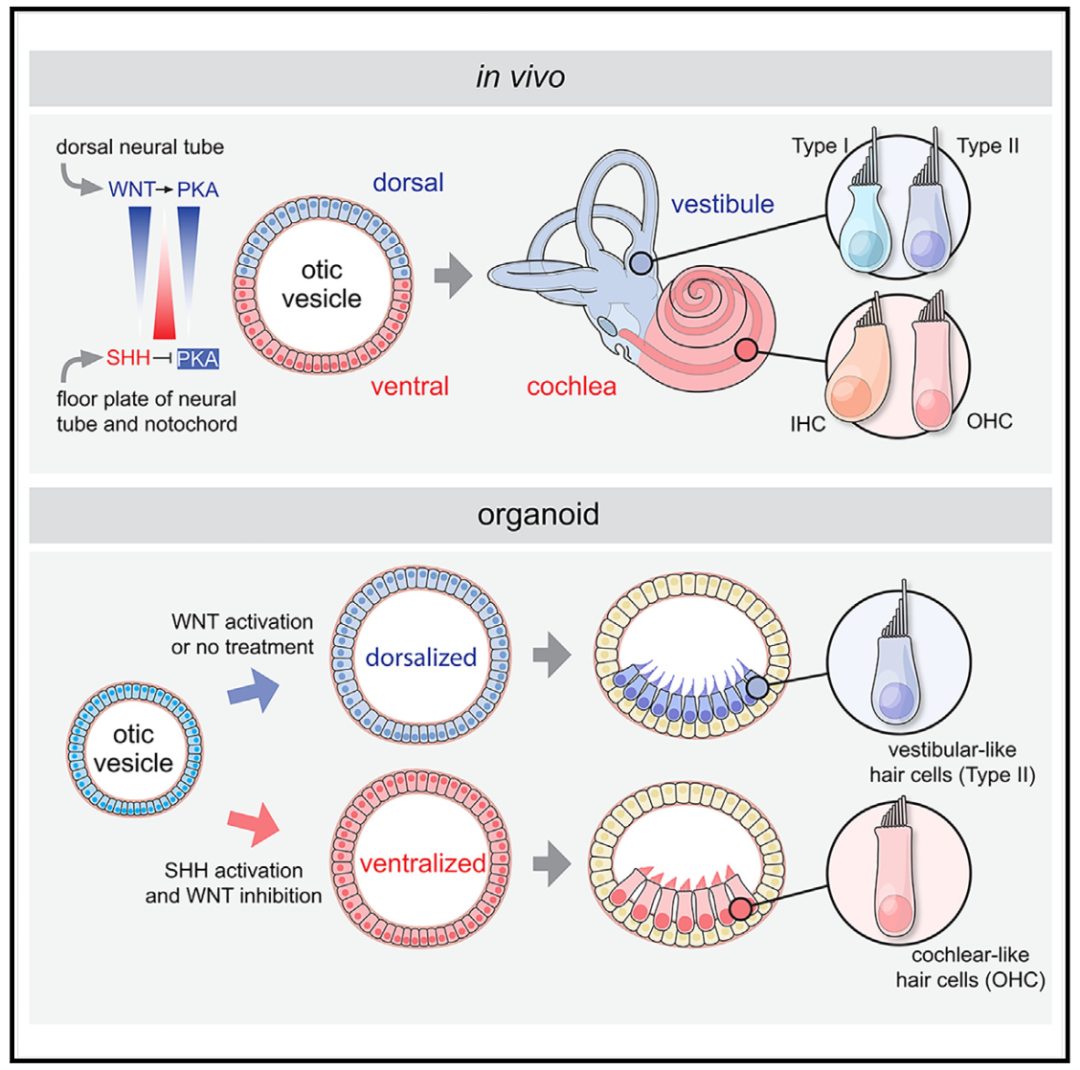
Figure 3. Working model of cochlear organoids
The scientists used 3D culture techniques of iPSCs to try to find key differentiation clues for cochlear replication. The researchers found that wnt signaling promotes gene expression in biological progenitor cells. Subsequently, the ventriculized progenitor cells differentiated into epithelial cells, whose morphology, marker expression, and functional properties were consistent with those of the internal and external hair cells of the cochlea. These results suggest that iPSCs differentiated progenitor cells are sufficient to drive the development of cochlear sensing organs and establish an unprecedented system to reconstruct human hearing organs.
The researchers have shown that the precise temporal regulation of the SH and Wnt pathways imparts pluripotent progenitors of the ventral ear type, some of which subsequently give rise to cochlear hair cells of both structural, transcriptional and functional types, inner and outer hair cells and mature hair cells. Organs grown in vitro can fully enter a stage similar to the third trimester of human fetal development. In addition, SCRA-SEQ analysis identified NR2F1 as a previously unrecognized key transcriptional pathway essential for cochlear and vestibular diversification. Further research is needed to elucidate the mechanisms of the cross-dialogue between transcriptional pathways and structural development, and to establish a way to control inner and outer hair cell production. The cochlear organ established in the study is considered a powerful human model that can be applied to study the biology of human cochlear development, elucidate the pathogenesis of inherited hearing loss, and identify therapeutic targets for the treatment of deep hearing loss.
Although the current stem cell treatment of hearing loss is still in the basic research stage, with the further development of more research projects, more and more meaningful data are accumulating, it is believed that in the near future, we can achieve more reliable treatment through mass cultivation of human cochlea, helping tens of millions of patients to remove hearing AIDS and return to a normal life full of laughter.
To sum up, by reproducing the key differentiation cues of cochlear tissue development, the author optimised the induction scheme of cochlear organoids by sequentially regulating Sonic Hedgehog and WNT signaling pathways, and promoted the development of hair cells with different morphology and the expression of markers, structural characteristics and electrophysiological characteristics in cochlear organoids. It is a comprehensive simulation of the human auditory system and provides an important tool platform for the development of the inner ear and the study of pathology.