
Source: Nankai Cell Engineering
Abstract: Type 1 diabetes mellitus (T1D) is a chronic autoimmune disease caused by congenital and specific immune responses to beta islet cells. Islet transplantation is a treatment for regulating plasma glucose in patients with T1D. However, islet transplantation is limited by low donor availability, high islet loss rate and immune rejection, and it is urgent to develop new techniques to improve the efficiency and survival rate of islet implantation and maintain the function of islets. Mesenchymal stem cells (MSCs) are pluripotent non-hematopoietic progenitor cells with high plasticity, which can ensure the function of islet cells in vivo and in vitro. In islet transplantation, co-transplantation of islets and MSCs is more effective than single islet transplantation. In addition, MSCs also have some potential in controlling graft rejection. MSCs benefit angiogenesis, suppress the immune response, and secrete growth factors necessary for islet survival and function, and can have a positive effect on islet cell transplantation by interacting with innate and adaptive immune system cells either through direct cell-cell contact or through their secretomes, including exosomes. In this paper, we describe multiple aspects of mesenchymal stem cells related to islet function and diabetes, discussing the current critical role of mesenchymal stem cells as cell managers capable of controlling the immune system to prevent rejection and promote endogenous repair.
Key words: islet cell transplantation; Type 1 diabetes; Mesenchymal stem cells
1 Introduction
type 1 diabetes (T1D) or juvenile diabetes is a chronic autoimmune disease that occurs when insulin-producing beta cells in the endocrine pancreas are gradually destroyed by immune cells. T1D is characterized by chronic inflammation of the islets and infiltration of immune cells, called insulinitis. Both hypoglycemia and hyperglycemia can lead to health complications. Therefore, T1D patients need to inject insulin subcutaneously to maintain normal blood sugar levels. Islet transplantation or whole pancreas transplantation have also been used to treat T1D, but these treatments require subsequent immunosuppressive therapy. Up to 80% of transplanted islets are lost before integration into the tissue due to acute inflammation induced by transplantation and the release of pro-inflammatory cytokines IL-1β, TNF-α, and IFN-γ. Allograft rejection and autoimmune recurrence also pose challenges to the success of islet transplantation. In addition, the limited supply of tissue is also a problem in islet transplantation.
Mesenchymal stem cells (MSCs) are pluripotent non-hematopoietic progenitor cells found in a variety of tissues, including bone marrow, adipose tissue, liver, and cord blood. They can differentiate into many cell types, including bone cells, fat cells, chondrocytes, endothelial cells, and muscle cells. The low immunogenicity and immunosuppressive properties of MSCs make MSCS a promising therapeutic tool for treating various autoimmune diseases, including T1D. Many studies have shown that mesenchymal stem cells can reduce the immune response after islet transplantation and improve the efficiency of islet transplantation by regulating immunity and promoting angiogenesis. This article will introduce the beneficial effects of mesenchymal stem cells on β cell function.
2 Islet transplantation for type 1 diabetes
In 2000, Shapiro et al. developed the Edmonton protocol for islet cell transplantation and introduced glucocorticoid-free immunosuppression protocol, which broke through the traditional treatment of T1D and brought hope for a complete cure of T1D. In 2006, Shapiro et al. published a follow-up of 28 patients treated with their islet cell transplantation at 36 locations worldwide. Under the Edmonton protocol, Shapiro et al. isolated islet cells from deceased donors and transplanted them into patients via portal vein injection. Of the participating patients, 58% were insulin-dependent at some point in the study despite being under good glycemic control, and 76% required some degree of insulin therapy 2 years after transplantation. In addition, this study illustrates the destructive effect of immunosuppressive drugs on islet cell function, and promotes subsequent research on single-drug immunosuppression. Immunosuppressive drugs are highly toxic in the hepatic portal vein, so it is very important to further study the alternative transplantation site of islets.
In 2022, Marfil-Garza et al. published the results of a 20-year study of islet cell transplantation at the University of Alberta in Edmonton, Canada, which involved 255 patients in Edmonton alone and was the largest cohort study of long-term outcomes after islet transplantation. Despite chronic immunosuppression, this study illustrates the long-term safety of islet cell transplantation. At 7.4 years of follow-up, 90% of patients remained alive, with a median survival time of 5.9 years for their islet grafts. Compared with patients with non-continuous graft survival, patients with continuous graft survival showed better insulin independence and better continuous glucose control.
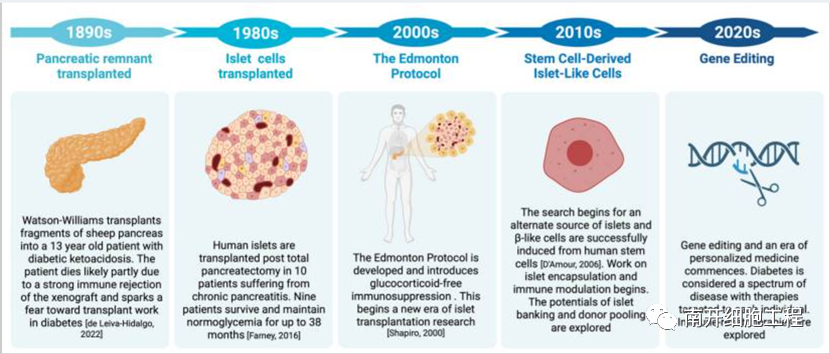
FIG. 1 Evolution of allogeneic islet cell transplantation from 1890 to the present
3 Mesenchymal stem cells
Multipotent stromal cells (MSC) are the source of cells that maintain homeostasis under physiological and pathological conditions during cell therapy. Unlike other stem cells, there are no ethical issues with the use of MSC. In addition, the therapeutic potential of MSC, known in preclinical animal disease models, has been tentatively validated in clinical trials.
Mesenchymal stem cells can be isolated from a variety of biological tissues, such as adult bone marrow (BM), adipose tissue, and prenatal tissue (such as umbilical cord and placenta), and are characterized by extensive proliferation, pluripotent, and homing/migration. Friedenstein was the first to isolate MSCS in mouse BM, and they showed immunosuppressive function both in vitro and in vivo. In addition, Davies and colleagues tested the difference between infusion of autologous MSCS (from host diabetic patients) or allogeneic MSCS from healthy donors and demonstrated that there were no functional differences between the two groups in terms of immunosuppressive activity, blood compatibility, or migration ability. There is evidence that the secretory group of mesenchymal stem cells plays a paracrine role and plays a protective role in acute pancreatitis.
Mesenchymal stem cells have been shown to ameliorate many autoimmune mediated diseases, including graft rejection, graft-versus-host disease, rheumatoid arthritis, and systemic lupus erythema. In addition, mesenchymal stem cells can promote wound healing and tissue regeneration. In various Settings, human mesenchymal stem cells have been shown to be beneficial for diabetes. More and more evidence shows that mesenchymal stem cells have positive effects on insulin-producing beta cells and islet survival, and islet co-transplantation with mesenchymal stem cells can increase the survival rate of islet grafts. The advantage of MSC transplantation is that it is well tolerated by patients with no obvious toxicity, but occasionally there are some adverse reactions, such as gastrointestinal and skin diseases. After mesenchymal stem cell transplantation, these cells can provide cytokines, chemokines, growth factors and other bioactive factors through differentiation into various cell phenotypes, enhance the proliferation of stem cells and tissue progenitor cells and inhibit immune response, and thus become a repair environment for damaged tissues and promote tissue regeneration.
4 Protection of islet function by mesenchymal stem cells
In vitro culture of human islets can lead to loss of function, dedifferentiation, senescence, apoptosis and necrosis. Isolation of islet cells from the body also has a certain probability of causing their death. Single cell transcription analysis of human islet after 3-6 days of isolation showed that the gene expression of insulin-positive beta cells was decreased, accompanied by an increase in the level of progenitor cell markers, indicating early dedifferentiation of isolated islet cells. Therefore, appropriate culture conditions need to be developed to maintain islet function for in vitro studies and islet preservation prior to islet transplantation. Several studies have shown that co-culture of islets with mesenchymal stem cells can maintain functional activity of islet cells in vitro and in vitro. The results show that mesenchymal stem cells provide factors to maintain β cell function and survival, and inhibit the spontaneous dedifferentiation of islet cells in vitro.
Yeung et al. observed that human mesenchymal stem cells protected human islet cells from the destruction of pro-inflammatory cytokines IFN-γ, TNF-α and IL-1β by secreting metalloproteinases 2 and 9. It has been demonstrated experimentally that MMP-2 and MMP-9 promote the immunosuppressive function of MSC by reducing the expression of IL-2R (CD25) on the surface of T cells. In mice with MMP-9 knockout, the pancreas and islets developed normally, but the response pathway to hyperglycemia was impaired, that is, the amount of insulin secreted by islets under hyperglycemia conditions decreased after MMP-9 knockout. This suggests that extracellular matrix turnover is important for the release of paracrine factors from the matrix.
In the treatment of T1D, MSC has shown an effective role in regulating fibrosis and tissue regeneration. In animal models of diabetes, combined transplantation of mesenchymal stem cells and islets is more effective than islet transplantation alone. Repeated bone marrow transplantation in diabetic mouse models can restore normal blood sugar and restore the pancreas to normal shape. Systemic administration of MSC in diabetic mice or rats can promote islet regeneration, increase endogenous insulin production, reduce blood glucose levels, reduce pancreatic inflammatory processes, and prevent kidney injury. Although some studies have shown that the paracrine function of mesenchymal stem cells contributes to the beneficial effects of mesenchymal stem cells, the efficiency of mesenchymal stem cell conditioned media is much lower than that of mesenchymal stem cell transplantation. When mesenchymal stem cells and islets were transplanted into diabetic animals, the beneficial effects of mesenchymal stem cells on islets were obviously observed.
Some mesenchymal stem cells produce factors that help maintain islet beta cell function and survival, so co-transplantation of islet cells with mesenchymal stem cells can improve the efficiency of islet transplantation.
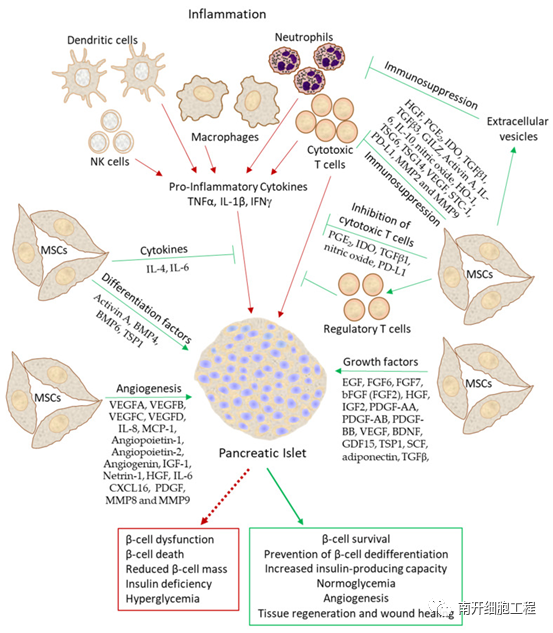
FIG. 2 Illustration of the beneficial effects of mesenchymal stem cells on islet beta cells
(The green arrow shows the beneficial effects of mesenchymal stem cells on beta cells, and the red arrow shows the harmful effects of inflammation on beta cells)
5 Discussion and prospect
In this paper, we first discuss islet cell transplantation as a treatment option for type 1 diabetes, which, despite its broad application prospects, is inevitably associated with chronic immunosuppression. In order to preserve the morphology and function of islet cells in vitro, and to deal with the problem of chronic immunosuppression, we discussed the protective effect of mesenchymal stem cells on islet β cells and the inhibition of autoimmunity. To date, in vivo studies have focused on whether transplantation of mesenchymal stem cells is accompanied by immunosuppressive therapy, which is effective in the short term and can also achieve long-term results in some patients with diabetes. Considering the data obtained from animal studies, it is desirable to combine mesenchymal stem cells with islet transplantation. However, the number of islet cells for transplantation is limited, so further research is needed to maintain the activity and function of islet beta cells in vitro. It is possible to optimize the composition of growth factors through in-depth exploration of MSC secretome to maintain β cell function, improve the efficiency of β cell differentiation in vitro, and maintain the function of islets in vitro.