
Source: School of Basic Medicine, Zhejiang University
The nucleolus is a variety of liquid phase condensates with liquid-liquid phase separation (LLPS). The typical nucleolus is a three-layer compartmentalized structure where many proteins locate and participate in the construction of fiber center (FC), dense fiber component (DFC), and particle component (GC) liquid stratification. Nucleolar phase separation is a crucial molecular event required for ribosome biogenesis, protein translation, and nucleolar stress response. However, its role in the fate determination of pluripotent stem cells is unclear. LIN28A is a highly conserved RNA binding protein that plays an important role in promoting somatic reprogramming and pluripotent state transition. Inhibition of let-7 microRNA maturation by LIN28A in the cytoplasm has been extensively studied in the past, but few studies have focused on the role of LIN28A in the nucleus.
On February 10, 2024, Zhang Jin research Group of Liangzhu Laboratory, Center for Stem Cell and Regenerative Medicine, School of Basic Medicine, Zhejiang University/The First Affiliated Hospital of Zhejiang University School of Medicine, The research group of Feng Yu from the School of Basic Medicine of Zhejiang University/Run Run Shaw Hospital Affiliated to the School of Medicine of Zhejiang University published a paper entitled "Dynamic nucleolar phase separation influenced by Nature Communications. non-canonical function of LIN28A instructs pluripotent stem cell fate Decisions ", In this study, we found for the first time that LIN28A can be used as a marker protein of nucleolar integrity. The non-classical phase separation function of LIN28A can regulate reprogramming, and the IDR mutation of LIN28A associated with Parkinson's disease plays an important role in nucleolar phase transition.
The study, in conjunction with Jin Zhang's research group, was published in the journal Protein & Cell on July 31, 2021 under the title "LIN28 Coordinately Promotes Nucleolar/Ribosomal Functions and Represses the The research paper "2C-like Transcriptional Program in Pluripotent Stem Cells" and the November 4, 2021, issue of Nature Communications entitled "rRNA Biogenesis. Regulates Mouse 2C-like State by 3D Structure Reorganization of Peri-Nucleolar Research papers at Heterochromatin are closely related and further elucidate the mechanism of nucleolar involvement in regulating fate determination of pluripotent stem cells from the perspective of nucleolar stress and phase separation.
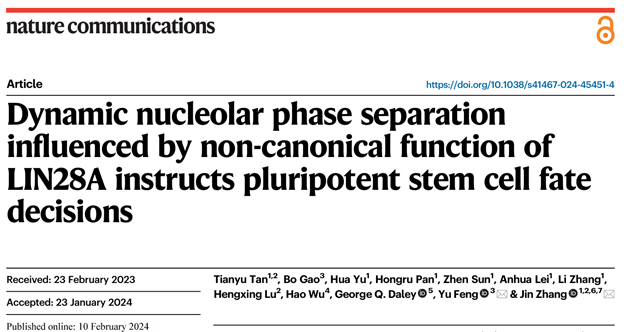
First, the researchers constructed E14 cell lines of mouse embryonic stem cells knocked in by eGFP-LIN28A and E14 cell lines of mouse embryonic stem cells overexpressed by eGFP-LIN28A. LIN28A was observed to be distributed in cytoplasm and nucleolus. LIN28A colocalized with NPM protein in the nucleolus granular component (GC) layer and coated with protein FBL in the dense fiber component (DFC) layer. This suggests that LIN28A protein in the nucleolus can be used as a marker of the nucleolus. At the same time, LIN28A in the nucleolus is sensitive to phase separation inhibitor 1, 6-hexanediol (HEX) treatment, and 1%HEX treatment leads to the obvious expansion and dispersion of LIN28A agglomerates in the nucleolus, the fluorescence intensity per unit area is significantly weakened, and the shape irregularness of LIN28A as calculated by the radius shape index formula is also significantly increased (FIG. 1).
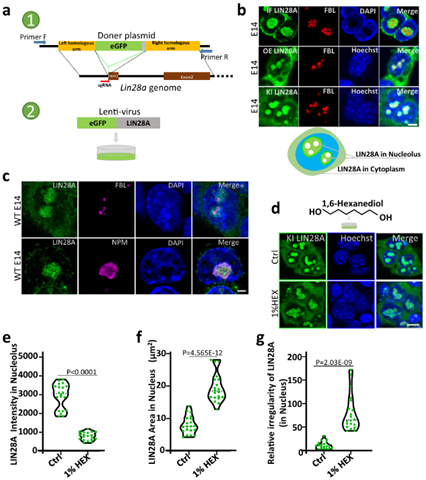
Figure 1 Localization of LIN28A in mouse embryonic stem cell E14
In mouse embryonic stem cells, the RNA-binding domain (RBD) and inner disordered region (IDRs) of LIN28A are required for nucleolar phase separation. LIN28A protein has five major domains: the N-terminal sequence (amino acids 1-38), which was predicted to be the intrinsically disordered region (IDR1) using the commonly used intrinsic disordered region evaluation site PONDR (http://pondr.com/); A cold shock domain (CSD) and a zinc finger domain (ZFD), which have been shown to bind RNA, are typical RNA binding regions; The region of flexible connection between CSD and ZFD (Linker, amino acids 113-136), which is predicted to be inherently disordered, is named IDR2; The C-terminal (amino acids 177-209) was also predicted to be a disordered region, which was named IDR3.
To determine the contribution of individual domains of LIN28A to nucleolar liquid phase separation, the researchers constructed truncated constitutions with N-terminal, C-terminal, N+ C-terminal, CSD (amino acids 39-112) and ZFD (amino acids 137-176) single-domain deletion. Then CRISPR/CAS9 technology was used to obtain monoclone of Lin28a knockout mouse embryonic stem cells E14, and then FBL-mCherry or NCL-eGFP fusion protein was statically expressed in Lin28a knockout cell line, and the corresponding protein was compared with eGFP. The mCherry fused truncated LIN28A was expressed into Lin28a knockout cell lines that had stable expression of FBL-mCherry fusion protein.
The results showed that Lin28a knockout resulted in the destruction of nucleolus, and the FBL changed from a wild type ring to a diffuse state. Overexpression of full-length LIN28A can save the abnormal morphology of FBL and restore the ring shape of FBL and NCL. Truncated bodies with deletion of N-terminal sequence (IDR1), C-terminal sequence (IDR3) or both N-terminal and C-terminal sequences of LIN28A could not save the nucleolar damage caused by LIN28A knockout, and FBL still showed an abnormal diffuse state. The absence of CSD or ZFD seriously damaged LIN28A nucleolar localization and FBL ring morphology, and the colocalization of LIN28A and FBL in the nucleolar was abnormal. In short, when the IDR or RBD of LIN28A is truncated, the DFC layer nucleolar protein FBL loses its "ring" structure, suggesting abnormal nucleolar phase separation stratification. The quantitative statistics of the typical morphology of FBL in several truncated somatic cell lines indicate that the RNA-binding region and the inner disordered region of LIN28A are necessary to maintain normal nucleolar phase separation.
In addition, we examined the fluidity of FBL in several LIN28A cell lines and found that overexpression of full-length LIN28A could reverse the weakened fluidity of FBL caused by Lin28a knockout, whereas FBL showed relatively low fluidity in truncated LIN28A cell lines. This result also confirms that the RNA-binding region and the inner disordered region of LIN28A are necessary to maintain normal nucleolar phase separation from the perspective of fluidity.
Next, in in vitro experiments, the researchers purified full-length and several truncated LIN28A recombinant proteins and found that only full-length LIN28A proteins could form droplets, and any loss of IDR and RNA-binding domains would eliminate LIN28A's ability to form droplets from liquid-liquid phase separation. The deletion of the N-terminal sequence of LIN28A, the deletion of the flexible connection region between CSD and ZFD, and the deletion of the C-terminal sequence of the three IDR deletions would cause the protein to form irregular aggregates.
In summary, the IDR and RNA-binding domains of LIN28A are critical for the formation of the phase separation of LIN28A itself and for the facilitation of nucleolar protein phase separation (Figure 2).
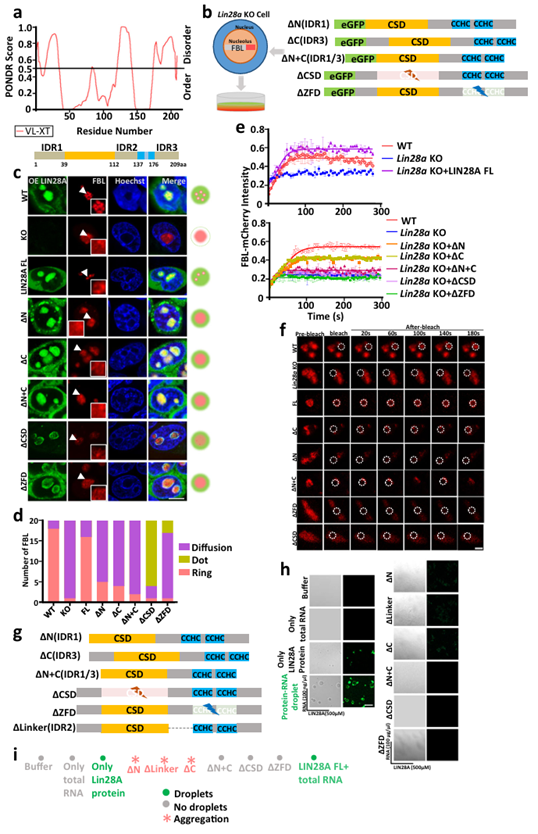
Figure 2 The RNA binding domain and IDRs of LIN28A are required for nucleolar phase separation
The key amino acids of IDRs are essential for the separation of LIN28A and nucleolar phase. The above experiments demonstrated that RNA binding domains and internal disorder regions are crucial for the phase separation of LIN28A and nucleolus. Proteins that can undergo liquid-liquid phase separation are characterized by the presence of IDR sequences. The IDR region is a repetitive sequence rich in a few amino acids, usually a region of low complexity that does not fold into a fixed three-dimensional structure. The RNA binding domain has been intensively studied in the context of LIN28A function. The authors were more curious about the function of the IDR region, which had not previously been looked at in LIN28A studies. Next, the researchers further refined the scope of the study down to the key amino acids necessary to give IDR phase separation properties. Phosphorylable S120, S200 serine residues, and R192 arginine residues associated with Parkinson's disease are all in the IDR region of LIN28A, and these three amino acids are conserved between humans and mice. Some studies have shown that serine phosphorylation and arginine affect phase separation by changing the charge of the protein. In order to further understand the role of these amino acids in the phase separation of LIN28A, we replaced serine with alanine, constructed a LIN28A mutant that mimics dephosphorylated S120A and S200A diamino acid mutations, named Mut LIN28A, and stably expressed the mutant in E14 cell lines knocked out by Lin28a. The FRAP results showed that Mut LIN28A was less mobile in the nucleolus and led to less mobile nucleolus protein FBL.
The researchers then constructed a single amino acid mutant that simulated dephosphorylated S120A LIN28A and a single amino acid mutant that simulated dephosphorylated S200A LIN28A, respectively. FRAP analysis showed that both of the two simulated dephosphorylated single amino acid mutant LIN28A proteins exhibited low mobility, while the FBL had low mobility in the nucleolus.
Recently, the loss-of-function mutant R192G LIN28A was identified in two patients with early-onset Parkinson's disease, and overexpression of wild-type LIN28A has been reported to promote the therapeutic potential of cultured neural stem cells in models of Parkinson's disease. However, there is no known mechanism in the field of phase separation to resolve mutation-induced developmental defects and phenotypes associated with midbrain dopamine neurons. FRAP analysis showed that the LIN28A protein with R192G single mutation was less mobile in the nucleolus, as was the FBL protein. The fluidity of all mutants in the cytoplasm was not affected.
In order to further confirm that the nucleolus destruction of LIN28A mutant is due to its weakened phase separation properties, a salvage LIN28A mutant was constructed by fusing exogenous FUS IDR (S120A-FUS IDR, S200A-FUS IDR, R192G-FUS IDR). The FUS IDR is recognized to drive phase separation. Notably, the fused IDR restored the morphology and phase separation ability of the LIN28A mutant.
In summary, key amino acids in the IDR region are necessary for LIN28A phase separation and for maintaining normal nucleolar phase separation (Figure 3).
Figure 3 Key amino acids of IDRs are essential for the separation of LIN28A and nucleolar phase
Nucleolar phase separation maintained by LIN28A is essential for the pluripotent transformation of mouse embryonic stem cells from naive to primed. Compared with LIF/2i culture, wild-type mESCs cultured with FGF2/Activin A medium expressed higher primed state marker genes Otx2, Fgf5 and Lin28a, and lower naive state marker genes Klf4, Nanog and Esrrb. Compared with wild-type mESCs, Lin28A-knockout mESCs cultured in FGF2/Activin A culture medium showed a sustained high level of naive state related gene Nanog and Klf4 expression. When full-length wild-type (WT) LIN28A was overexpressed in LIN28A knockout cells, cells cultured in FGF2/Activin A medium restored their ability to transition to primed state. This suggests that LIN28A can promote the pluripotent transformation of mESCs from naive to primed.
When the phosphorylated Lin28a mutant (Mut LIN28A) was overexpressed in LIN28A knockout cells, NANOG immunostaining and fluorescence intensity statistical analysis showed that the Mut LIN28A mutant cells continued to express Nanog gene at A high level in FGF2/Activin A medium. Tends to maintain a naive state. Phosphorylated deletion of LIN28A (S120A and S200A) does not promote ESC exit from naive state to primed state. The three single amino acid mutants, S120A, S200A and R192G LIN28A, also lost their function of promoting mESCs to exit from naive state and enter primed. However, after fusion with FUS IDR, the NANOG fluorescence of the three mutants decreased in FGF2/Activin A medium. mESCs can enter primed state smoothly. These results suggest that LIN28A phase isolation is essential for the pluripotent transformation of mouse embryonic stem cells from naive to primed.
STED ultra high resolution imaging showed that FBL formed large typical "ring" structures in wild-type mESCs in the primed state, indicating that DFC units in the primed state were more developed. The absence of LIN28A will lead to the non-obvious ring structure of FBL, the abnormal morphology of NPM, the inability of FBL to infiltrate into the pore of NPM, and the disorder of nucleolar stratification. This abnormal FBL morphology and nucleolar stratification did not improve during the transition from naive state to primed state. These results suggest that LIN28A helps to correctly stratify nucleolus and maintain normal phase separation during the transition from naive state to primed state.
In summary, during the transition from a naive pluripotent state to a primed pluripotent state, the morphology and function of the nucleolus become more mature. Lin28a-mediated phase separation plays an important role in the nucleolar remodeling process and subsequent cell fate determination (Figure 4).
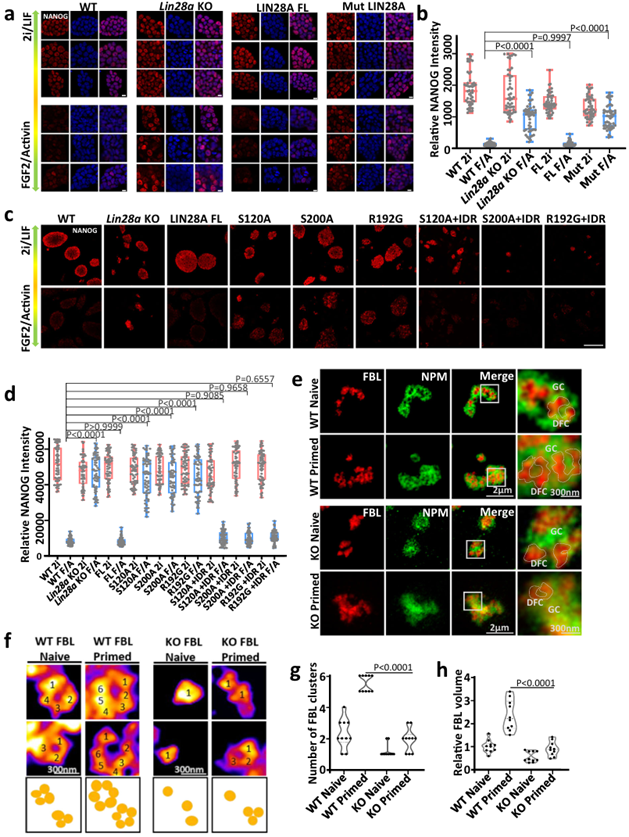
FIG. 4 Nucleolar phase separation maintained by LIN28A is essential for pluripotent transformation of mouse embryonic stem cells from naive to primed
LIN28A phase separation is essential for somatic cell reprogramming. Therefore, the researchers wondered whether the phase separation of LIN28A in the nucleolus would affect the efficiency of reprogramming. In mouse somatic cell reprogramming experiments, single amino acid mutation in the IDR region of LIN28A resulted in reduced reprogramming efficiency. The LIN28A mutant after the fusion of the FUS IDR rescued the reduced reprogramming efficiency and had a similar reprogramming efficiency as the wild type.
The authors further identified the nucleolar status of mouse MEF cells and iPSCs, and STED imaging revealed significant differences in nucleolar morphology and stratification between MEF cells and mouse iPSCs. In MEF cells, NPM is irregularly shaped. In iPSCs, NPM has a circular "lotus root" structure with small holes and is co-located with LIN28A. The regularity of GC layer shape was quantified using the radius index (Boyce-Clark semidiameter index). Compared with MEF cells, GC layer morphology shows a higher degree of regularity in iPSCs.
In summary, in the context of mouse somatic cell reprogramming, IDR region-mediated LIN28A phase separation is crucial for nucleolar remodeling during mouse somatic cell reprogramming, and is related to the efficiency of programming. The process of reprogramming is also the process by which nucleolar layering becomes clear (Figure 5).
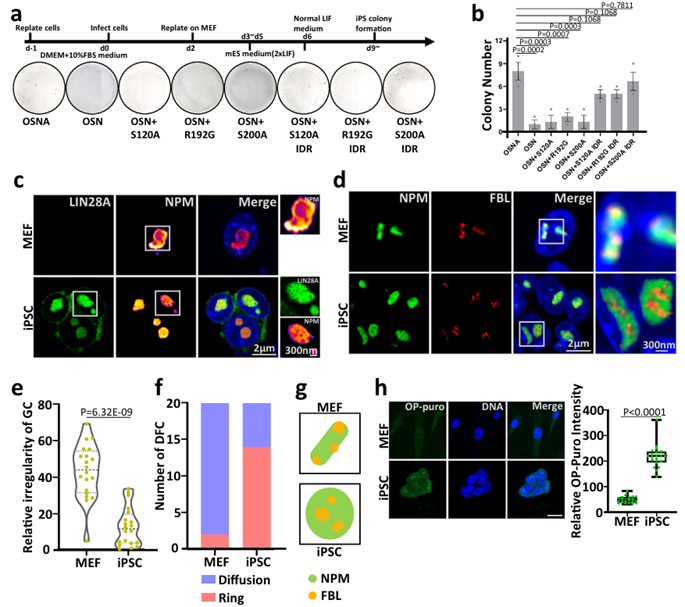
Figure 5 LIN28A phase separation is essential for somatic cell reprogramming
In summary, this study confirmed that LIN28A helps to improve nucleolar layering, which promotes the pluripotent transformation of mouse embryonic stem cells from naive to primed. Moreover, LIN28A phase separation in the reprogramming system plays a role in nucleolar remodeling, thus promoting reprogramming (Figure 6).
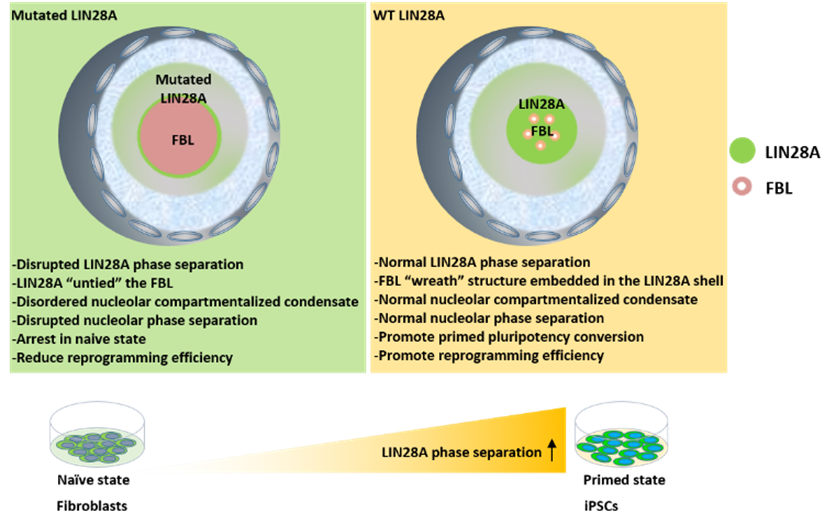
FIG. 6 Model of the role of LIN28A phase separation in maintaining nucleolar integrity and determining cell fate