
Source: GK Green Key Biotechnology
Professor Felix B. Engel of the University of Erlangen published the paper "Direct 3D-Bioprinting of hiPSC-Derived Cardiomyocytes to Generate" in Advanced materials Functional Cardiac Tissues, "they proposed a direct 3D bioprinting method to insert cardiomyocytes derived from human induced pluripotent stem cells (hiPSC) into collagen hyaluronic acid ink to produce centimeter-sized functional rings and ventricle-shaped heart tissue in an accurate and repeatable manner, and ultimately, The printouts are capable of performing heart-like functions, can be cultured for at least 100 days and respond to drug stimuli.
WHAT -- What are hiPSC derived cardiomyocytes?
Hipsc-derived cardiomyocytes are cardiomyocytes (HiPSC-CMS) differentiated from human induced pluripotent stem cells (hiPSC) that exhibit properties such as contraction and response to electrical signals. Cardiomyocytes derived from hiPSC have potentially important applications, especially in heart disease treatment and research, for disease modeling, drug discovery, personalized medicine and other applications.
WHY -- Why do experiments with direct bioprinting?
One goal of biofabration is to design tissues, parts of organs, to study or treat diseases for which current treatments are limited. The main biomanutrients currently used, one is cell-free 3D scaffolds, but a major disadvantage is the difficulty of colonizing cells, especially in an organized manner. Another method is to cast hydrogels containing cells, however this method does not allow the fabrication of hierarchies. At present, 3D bioprinting technology is promising to solve the main bottlenecks and current problems faced by traditional bioprinting, 3D bioprinting can ensure the uniform distribution of cells throughout the construction process, can directly manufacture tissues with physiological functions, allow the manufacture of complex biological structures that are difficult to achieve with traditional methods, and can customize personalized tissue structures according to specific needs.
HOW - The authors used a three-step, two-component scheme to produce gelatin/Arabic gum particles by complex condensation.
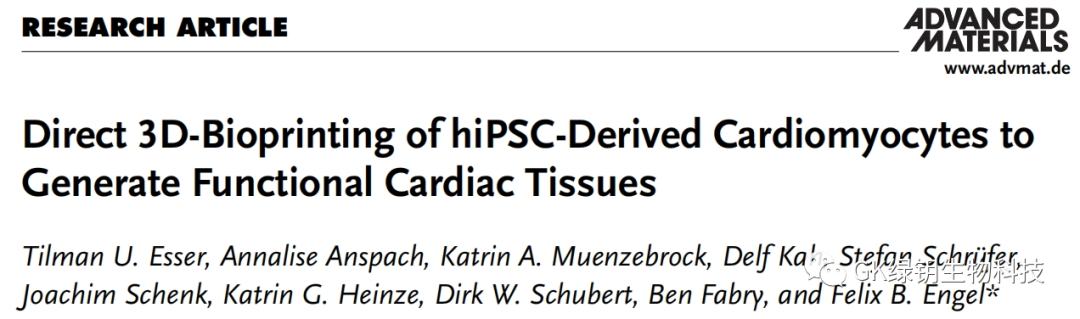
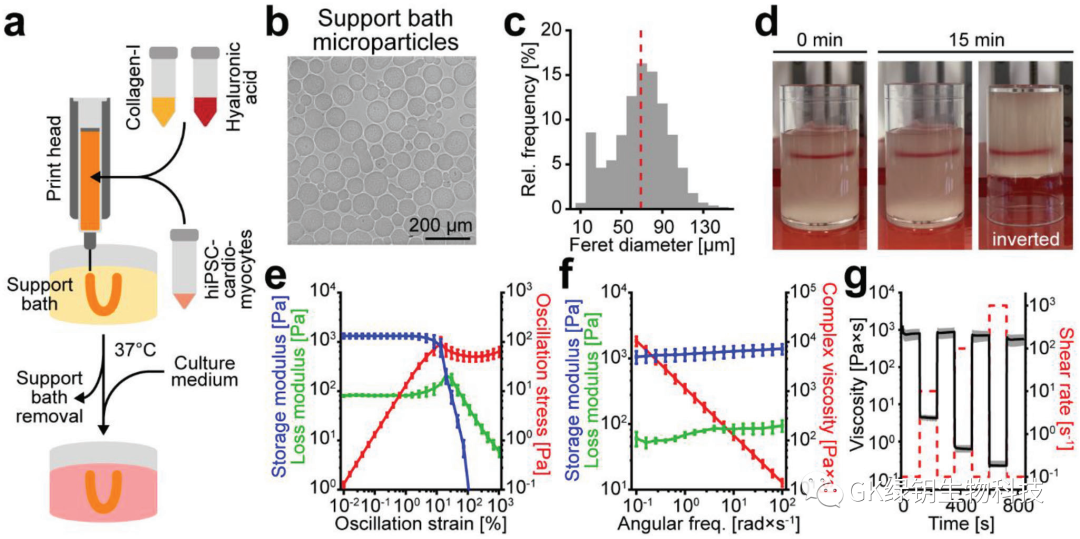 FIG. 1 The compacted particles exhibit shear thinning and self-healing properties
FIG. 1 The compacted particles exhibit shear thinning and self-healing properties
The particles are mainly round, and some are elliptical. Through centrifugal pressure, the particles form a support bath with self-healing properties, allowing the printing nozzle to pass freely. The dye in the support bath can remain in place even if the container is inverted. To confirm these observations, the authors performed rheological analyses. The results show that in the whole frequency range of the test, the support bath exhibits solid rheological behavior and shear thinning behavior at low strain, and the support bath exhibits near-instantaneous and complete recovery behavior at alternating shear rates. Taken together, the authors developed a simple method to produce a support bath with self-healing properties that can be used for gel printing.
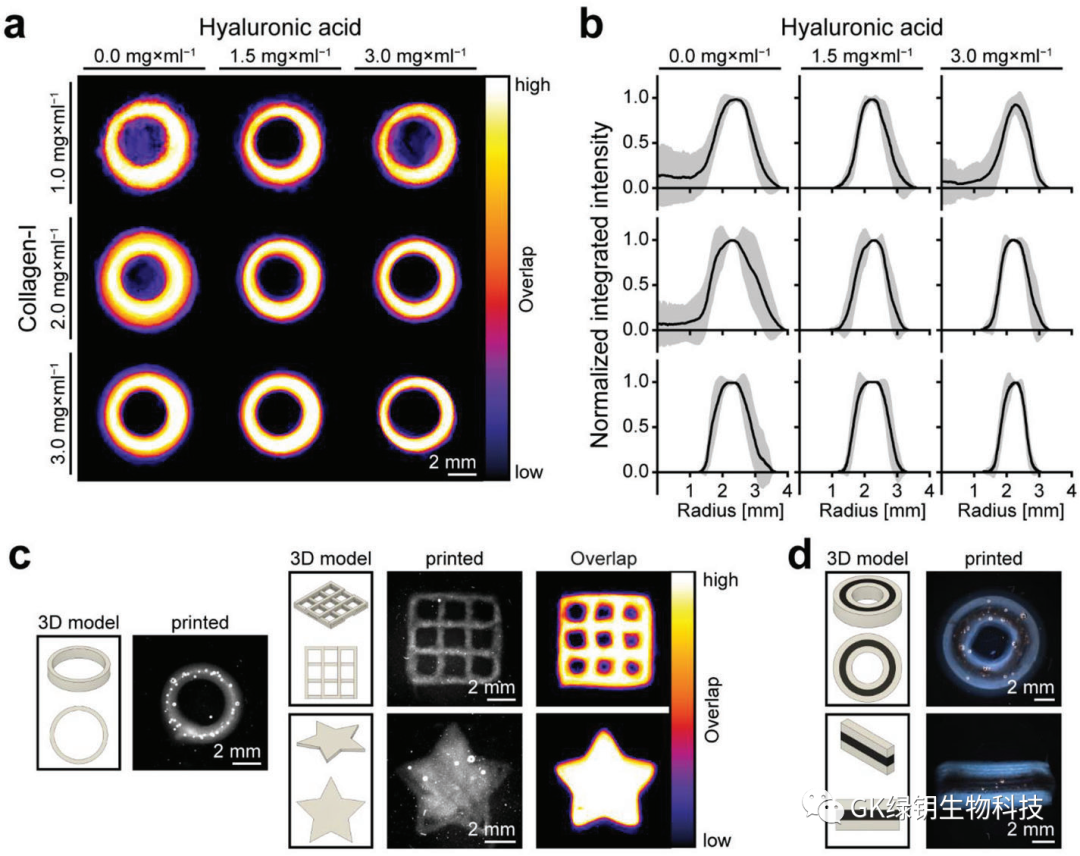 FIG. 2 Collagen/hyaluronic acid inks stabilize the print structure
FIG. 2 Collagen/hyaluronic acid inks stabilize the print structure
The authors evaluated the printability of collagen in the aforementioned support bath by printing acellular ring structures with collagen inks in different concentrations. In addition, hyaluronic acid (HA) was added to the collagen to improve the printability of the bioink. To determine the uniformity of the print rings and the repeatability of the printing process, the team generated overlapping maps from images of multiple print rings to evaluate. As the collagen content increased, the overlay map showed a more uniform print ring with a smoother border. In addition, as collagen concentrations increased and hyaluronic acid was added, the intensity spectrum showed smaller differences and became more defined. The authors ultimately selected a biochain consisting of 3.0 mg/ml collagen-I and 3.0 mg/ml HA (CollHA3-3) for further study, which has the highest reproducibility and the best control of extrusion speed, and the printed ring structure has a thin wall thickness.
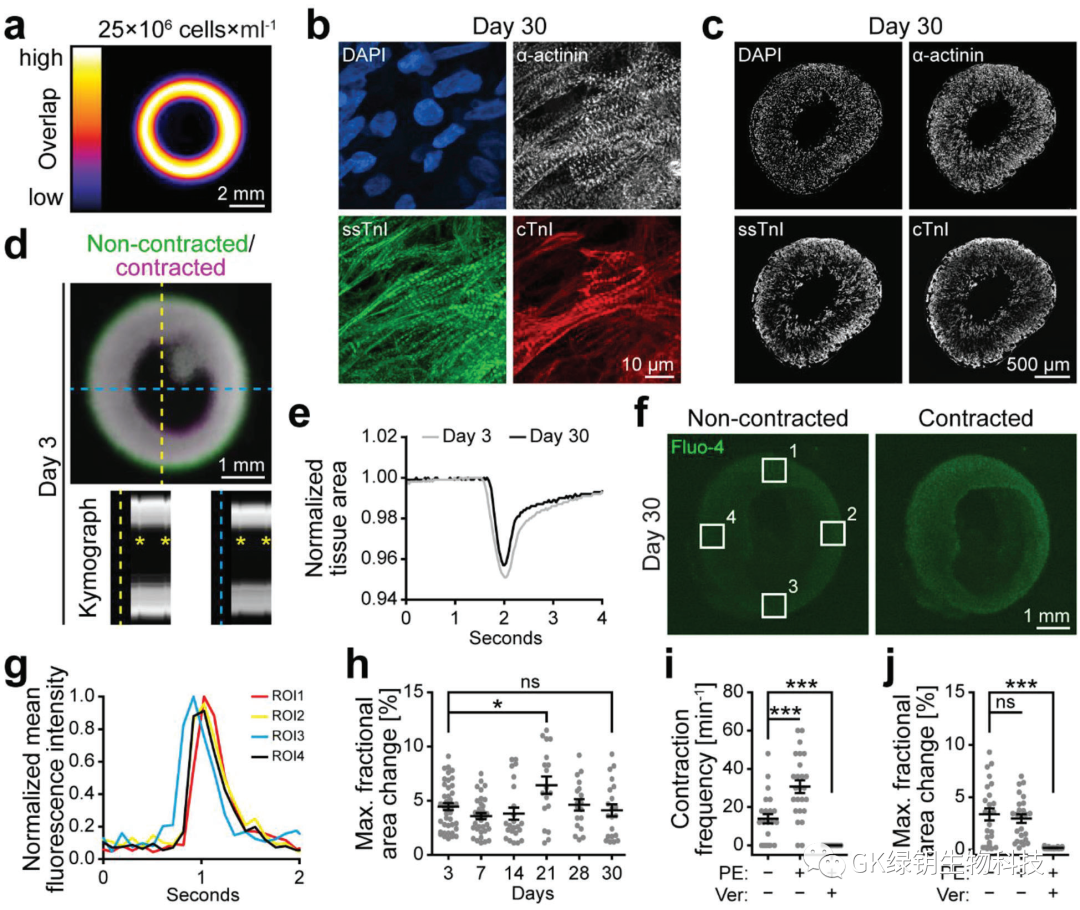 FIG. 2 Printing of HIPSC-derived cardiomyocytes forming functional heart tissue
FIG. 2 Printing of HIPSC-derived cardiomyocytes forming functional heart tissue
In order to produce hierarchically structured tissues or organs, it is important to control the distribution of cells. To assess whether the addition of cells would affect the printability of the ink, the authors added 25 Mio/ml hiPSC-CMs to the CollHA3-3 ink for printing ring tissue. The results show that the structure of the printed ring is stable and shows good uniformity. The printed tissue and the drip cast formed tissue were stained after tissue construction (day 0) and 7 days after manufacturing (day 7). The results showed that there was no significant difference in the vitality of the cast and printed tissues at any point in time. On day 30, the printed hiPSC-CMs showed typical myosynthetic α-actin stripes, as well as slow skeletal troponin-I (ssTnI) and cardiac troponin-I (cTnI). Forty-five of the printed tissues had shown spontaneous concentric contraction by day 3. The increase and decrease of Fluo-4 fluorescence intensity reflected the simultaneous inflow and outflow of calcium in the printed tissue during the contraction cycle. Measuring fluorescence intensity in different regions can identify pacemaker centers whose behavior shows high synchronization over time, with a delay of about 100 ms(1 frame) in reaching peak fluorescence intensity in distant regions. This is further evidence that hiPSC-CMs forms an interconnected network within the printing organization.
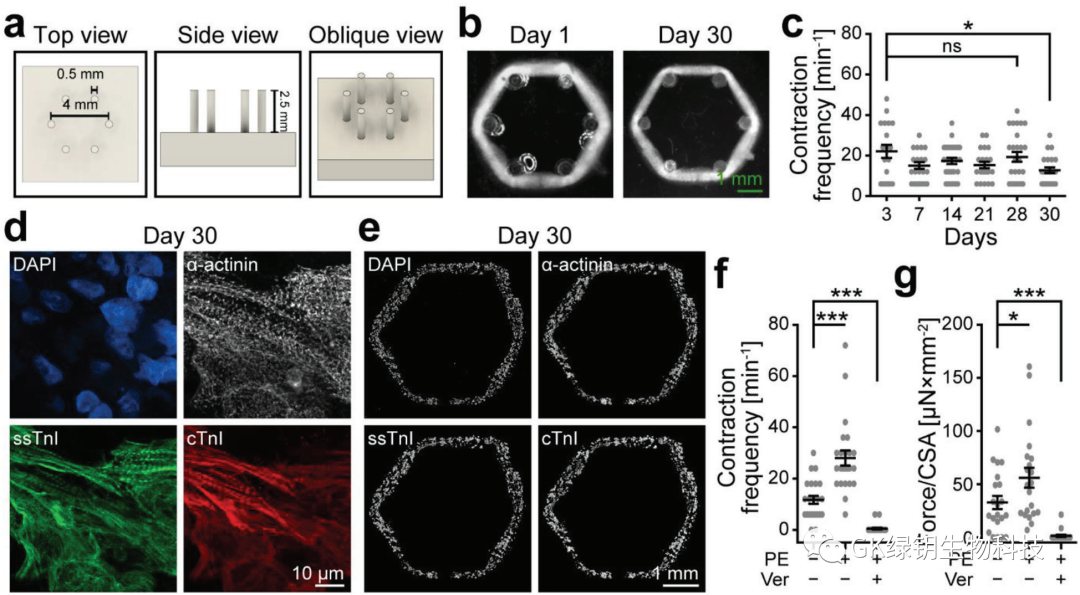 FIG. 4 Printed heart tissue exerts a homogenous force on passive resistance
FIG. 4 Printed heart tissue exerts a homogenous force on passive resistance
To test whether the print ring could exert concentric circular forces similar to those of the heart, the authors designed and manufactured an array of six flexible columns. On day 1, the printed rings were transferred to the column array and compacted around the columns, cultivated until day 30. During the contraction cycle, the printed rings were able to synchronously offset all six columns, indicating that they were able to exert similar concentric circle forces on the resistance.
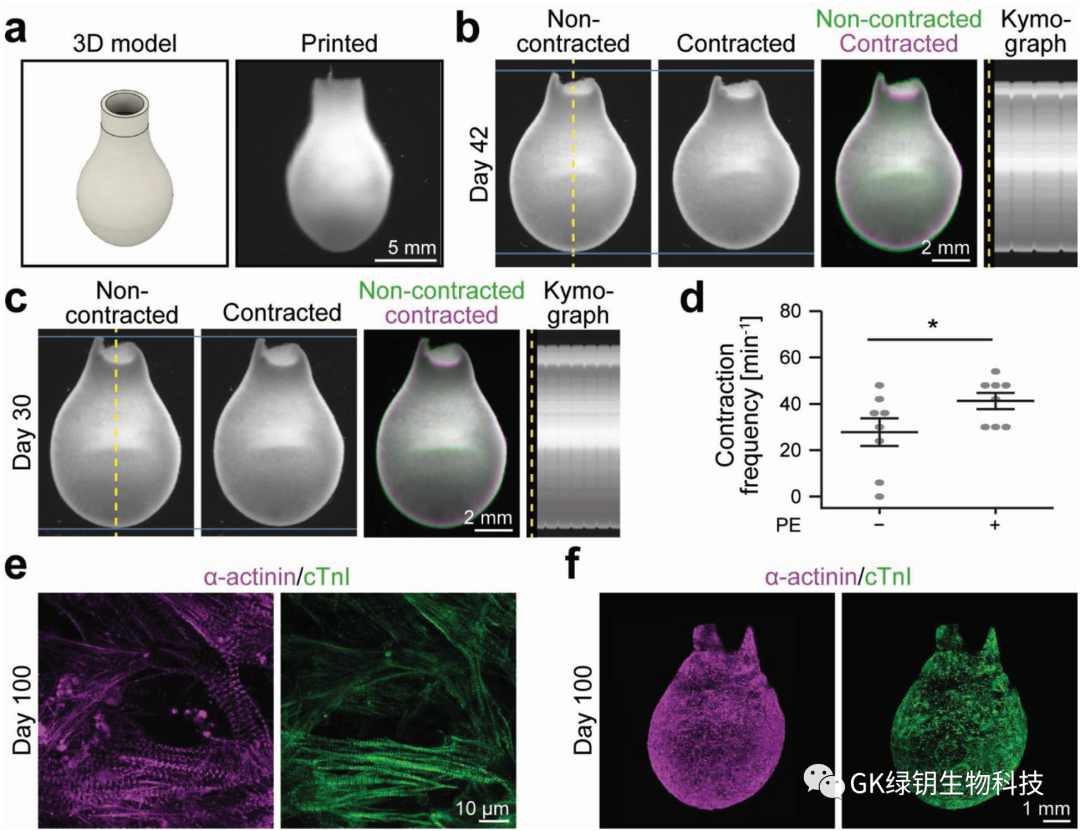 FIG. 5 3D bioprinted functional ventricular model
FIG. 5 3D bioprinted functional ventricular model
The authors designed and printed a ventricular model with a height of 14 mm and a diameter of 8 mm at the widest point to test whether their method could enable 3D bioprinting of ventricular function models of the human heart. After 7 days of manufacture, the printed ventricle was stable in shape and spontaneously contracted synchronously, expressing products related to ventricular function. Finally, the author shows that the culture time of the printed model can reach at least 100 days. There is hope that in the future, artificial 3D bioprinted cultured heart ventricles can be printed and grown for human use.
Summary: The authors achieved the direct printing of hiPSC-CMs embedded with collagen-based bioinks to generate functional heart tissue. The following experimental results support this conclusion. 1. hiPSC-CMs embedded in collagen /HA ink can be accurately and repeatably 3D bioprinted into ring and ventricle-shaped heart tissue and form interconnected networks; (2) The printed tissue exhibits spontaneous and synchronous contractions that last for several months, the frequency of which can be modulated by drug stimulation; 3, the printing tissue can resist passive resistance shrinkage. The authors' approach offers ideas for making advanced drug screening models, tissue transplants, and even engineered hearts that might one day be used for organ transplants.
Original link:https://doi.org/10.1002/adma.20230591