

Recently, with the cooperation of long March Hospital, Renji Hospital and Chinese Academy of Sciences on Cell Discovery, a case of type 2 diabetes treated with islet tissue derived from embryonic stem cells in vivo has been completed with excellent results.
The patient, a 59-year-old male with a 25-year history of type 2 diabetes, developed end-stage diabetic nephropathy and received kidney transplantation in June 2017. the blood glucose level was poorly controlled since November 2019, the blood glucose level was between 3.66 and 14.60mmol/L, and the average blood glucose deviation (MAGE) was 5.54mmol/L.
Due to the main concern about hypoglycemia and considering the adverse effects of poor blood glucose control on the long-term survival of the donor kidney, the patient agreed to use intrahepatic transplantation of islet tissue derived from embryonic stem cells in vivo.
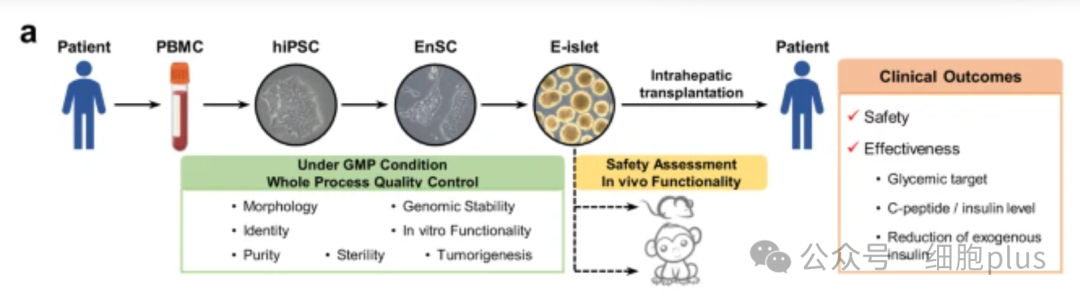
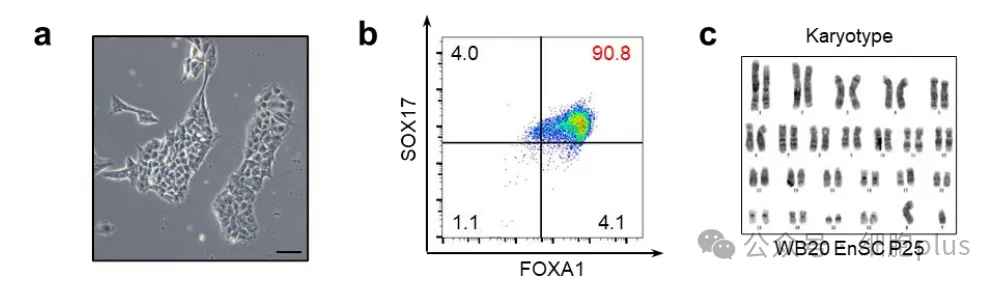
As can be seen from the image above, the endoderm progenitor cells (EP) were established by inducing pluripotent stem cells from autologous peripheral blood, and then derived into islet tissue.
Previous studies have confirmed that no tumor formation or cystic / ductal structure indicating cell proliferation was detected when endodermal stem cell EnSC or endodermal stem cell derived islets were transplanted into immunodeficient animals.
The patient received percutaneous transhepatic portal vein transplantation and 1.2 million IEQ islet tissues were given, in accordance with the regulatory guidelines for clinical islet transplantation registration.
Endocrine function and diabetes specific parameters were examined by mixed Dietary tolerance Test (MMTT) at baseline, 4, 8, 12, 16, 20, 24, 36 and 48 weeks and at specified time points after the designated visit, and blood glucose control was measured using a 24-hour real-time continuous blood glucose monitoring system (CGM).
During the follow-up of 116 weeks (nearly two and a half years), no tumor formation was detected either by MRI in the upper abdomen or by the measurement of serum tumor-associated antigen markers.
Three major clinical outcomes were monitored during the first 116 weeks, namely, blood glucose targets, reduction of exogenous insulin, and fasting and meal-stimulated circulating C-peptide / insulin levels.
As early as the second week after transplantation, significant changes in blood glucose control were observed. MAGE decreased from 5.50 mmol/L to 3.60 mmol/L, and the titer increased rapidly from 56.7% to 77.8%.
During the same period, overtime (TAR) decreased by 55% from the baseline, while severe hyperglycemia (> 13.9 mM) and hypoglycemia (< 3.9 mM) disappeared completely.
Between weeks 4 and 12, a significant decrease in dynamic average blood glucose fluctuation (from 5.50 to 2.6 mmol/L) and a steady increase in TITR (from 81% to 90%) were observed.
After the 32th week, the titer of the patient easily reached 99% and has been maintained ever since, while the gold standard of blood glucose variability, MAGE, dropped from 5.50 mM to 1.60 mM.
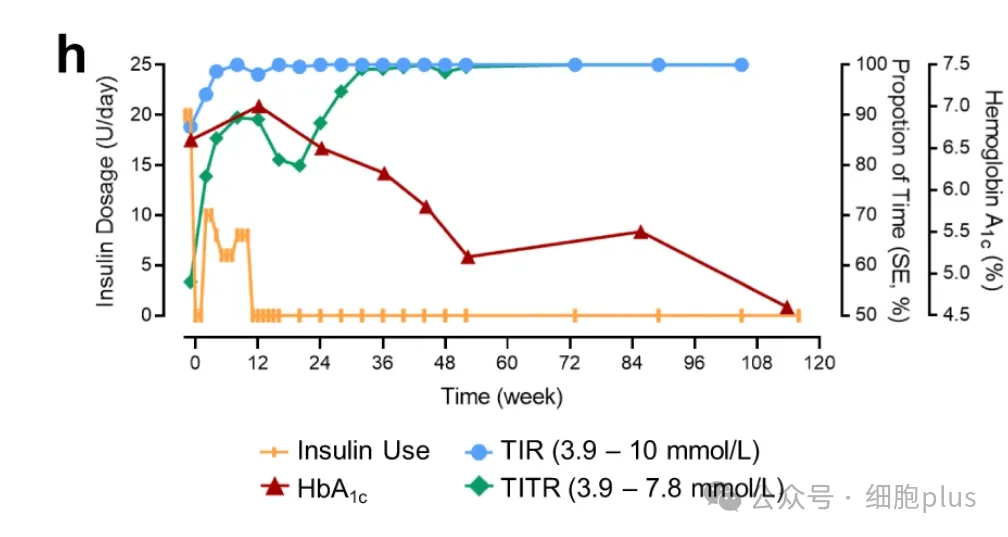
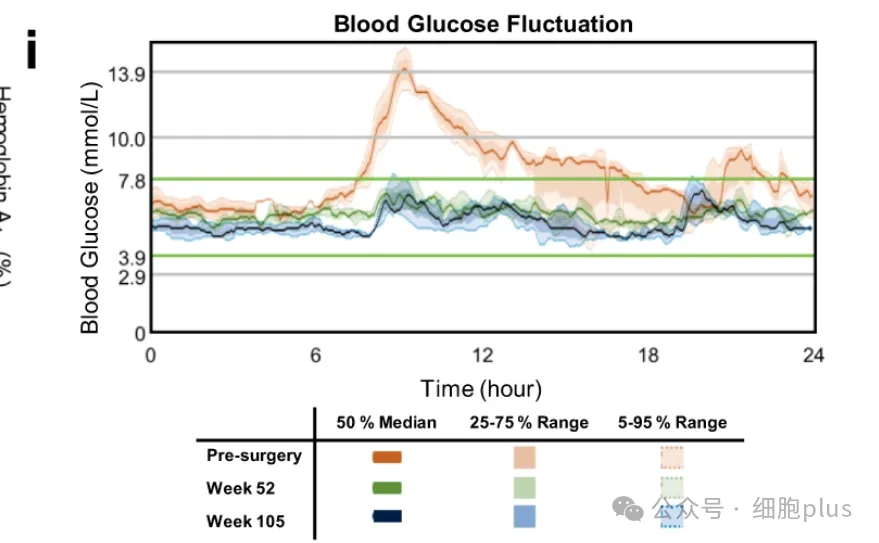
Importantly, no episodes of hypoglycemia or severe hyperglycemia were observed throughout the follow-up period of 116 weeks after operation. In addition, MMTT showed that the blood glucose variability tended to be stable after operation, showing a significant decrease in fasting blood glucose concentration and postprandial blood glucose concentration (the maximum was 21.3mM at baseline and 9.1mM at 105th week). Hemoglobin A1c levels decreased from 6.6% (baseline) to 5.5% (week 85) and 4.6% (week 113).
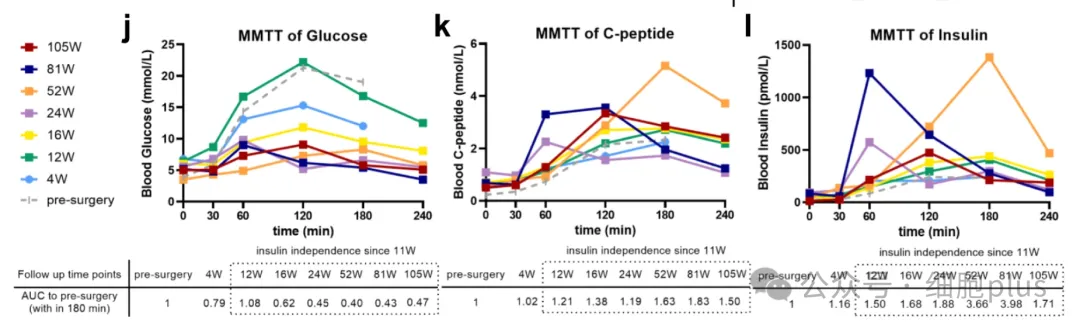
Compared with the preoperative level, the average fasting C-peptide level after operation (0.68 nmol/L) increased by 3 times.
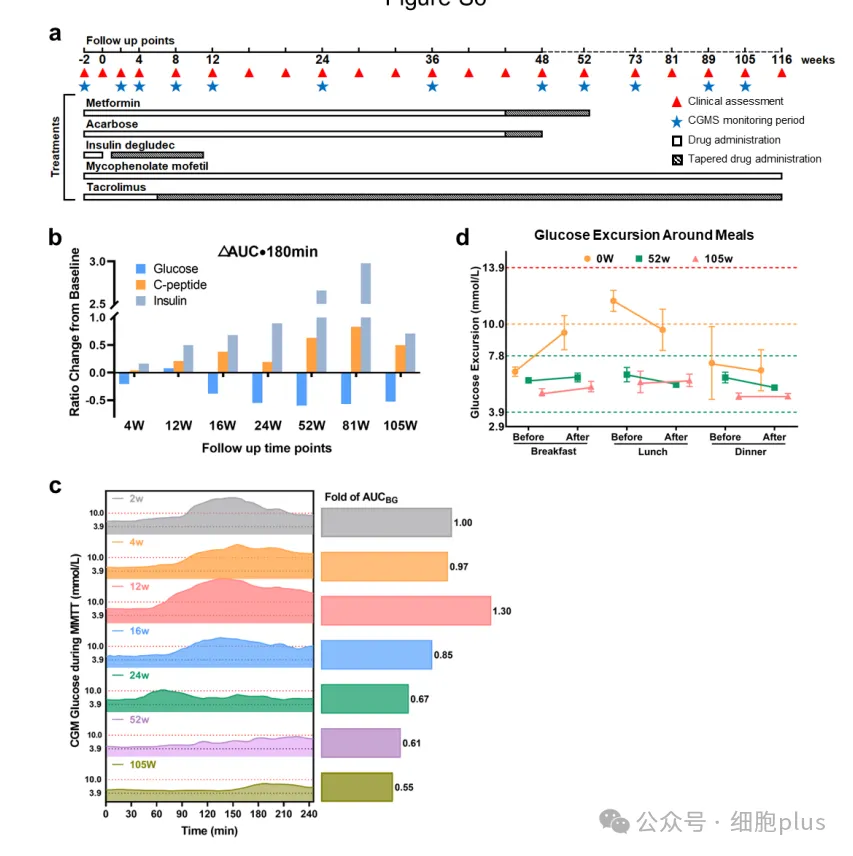
It is worth noting that the demand for insulin gradually decreased until the end of week 11, and oral antidiabetic drugs gradually decreased from week 44 to week 48 (acarbose) and week 56 (metformin).
In general, this is the first human tissue replacement therapy for T2D patients with impaired islet function treated with autoderived islets. Data from the first 27 months showed a significant improvement in blood glucose control and provided the first evidence that stem cell-derived islet tissue could save islet function in patients with advanced T2D.
The graft was well tolerated and there was no tumor formation or serious graft-related adverse events.