
On October 22, 2024, researchers from the Department of Biomedical Sciences, School of Medicine, Seoul National University, South Korea, published a research paper titled "Differentiating viscous sensory ganglion organisms from induced pluripotent stem cells" online in the journal Nature methods.
This study successfully established a new protocol for the differentiation of visceral sensory ganglion organoids (VSGOs) from human induced pluripotent stem cells (iPSCs). These VSGOs not only exhibit typical visceral sensory nervous system (VSNs) markers, but also reveal their consistency with natural VSNs in terms of heterogeneous molecular characteristics and development trajectories through single-cell RNA sequencing (scRNA-seq) technology. The research team further integrated VSGOs with human colon organoids (HCOs) on a microfluidic chip to build an in vitro model that simulates the gut-nerve-brain axis, particularly for Alzheimer's disease (AD) research.
Studies have found that VSNs may promote the spread of gut-derived amyloid (Aβ) and tau proteins to the brain through APOE4-and LRP1-dependent methods, providing a new perspective for understanding the pathogenesis of diseases such as AD. In addition, this study also expanded to use iPSCs from AD patients, and the experimental results showed a strong correlation with clinical data, further confirming the important value of this study in translational medicine and disease model research.
01 Background

When studying nervous system diseases, we have found that the visceral sensory nervous system (VSNs) plays a key role. These nerves originate from the superior branchial plate (EpP) in the embryo and can sense intestinal signals and transmit them to the brain through the vagus nerve. They are crucial to the function of the gut-nerve-brain axis.
In recent years, with the deepening of understanding of the relationship between the intestinal microbiome and nervous system diseases, people have paid increasing attention to the role of this axis in neurodegenerative diseases such as Alzheimer's disease (AD). Although there have been studies that manipulate the gut microbiome to reduce pathological changes in mouse models of neurological disease, most of these studies rely on in vivo models, limiting in-depth research into the gut-vagus-brain axis.
In order to simulate this axis in vitro and study its role in neurodegenerative diseases, researchers attempted to use induced pluripotent stem cells (iPSCs) to differentiate into visceral sensory ganglionic organoids (VSGOs). VSGOs can express a variety of molecular markers identical to VSNs, and single-cell RNA sequencing (scRNA-seq) reveals the heterogeneous molecular characteristics and development trajectories of VSGOs similar to native VSNs.
However, although some progress has been made in the induction of differentiation of VSGOs, there are still some gaps and challenges in current research. For example, how to more accurately simulate the in vivo environment to promote the maturity and function of VSGOs, and how to effectively integrate these organoids with intestinal organoids (HCOs) to build an in vitro model that can simulate the gut-nerve-brain axis. The so-called "Organs-on-a-Chip" model is a hot and difficult topic in current research.
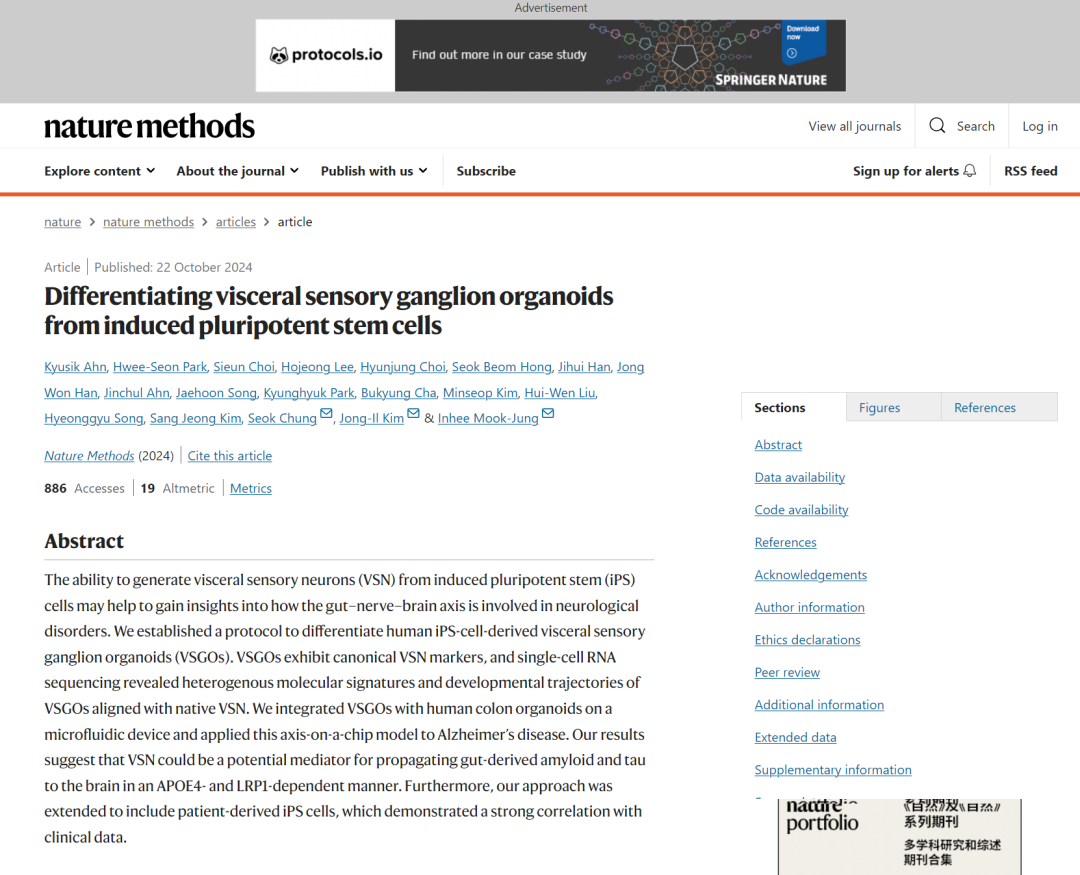
Differentiation of OEPD and EpP from iPS cells
02 Research steps

Against this background, researchers began research.
When exploring the role of the visceral sensory nervous system (VSNs) in neurodegenerative diseases, a core scientific question emerges: "Can VSNs serve as a medium for the spread of gut-derived pathological proteins to the brain?"
Based on current understanding of the gut-nerve-brain axis, researchers have proposed a hypothesis that VSNs may play a key role in the spread of gut-derived amyloid Aβ and tau proteins to the brain. To test this hypothesis, the researchers adopted an innovative research idea, which is to induce pluripotent stem cells (iPSCs) to differentiate into VSGOs and integrate them with colon organoids (HCOs) on a microfluidic chip. An in vitro model was built to simulate the gut-nerve axis.
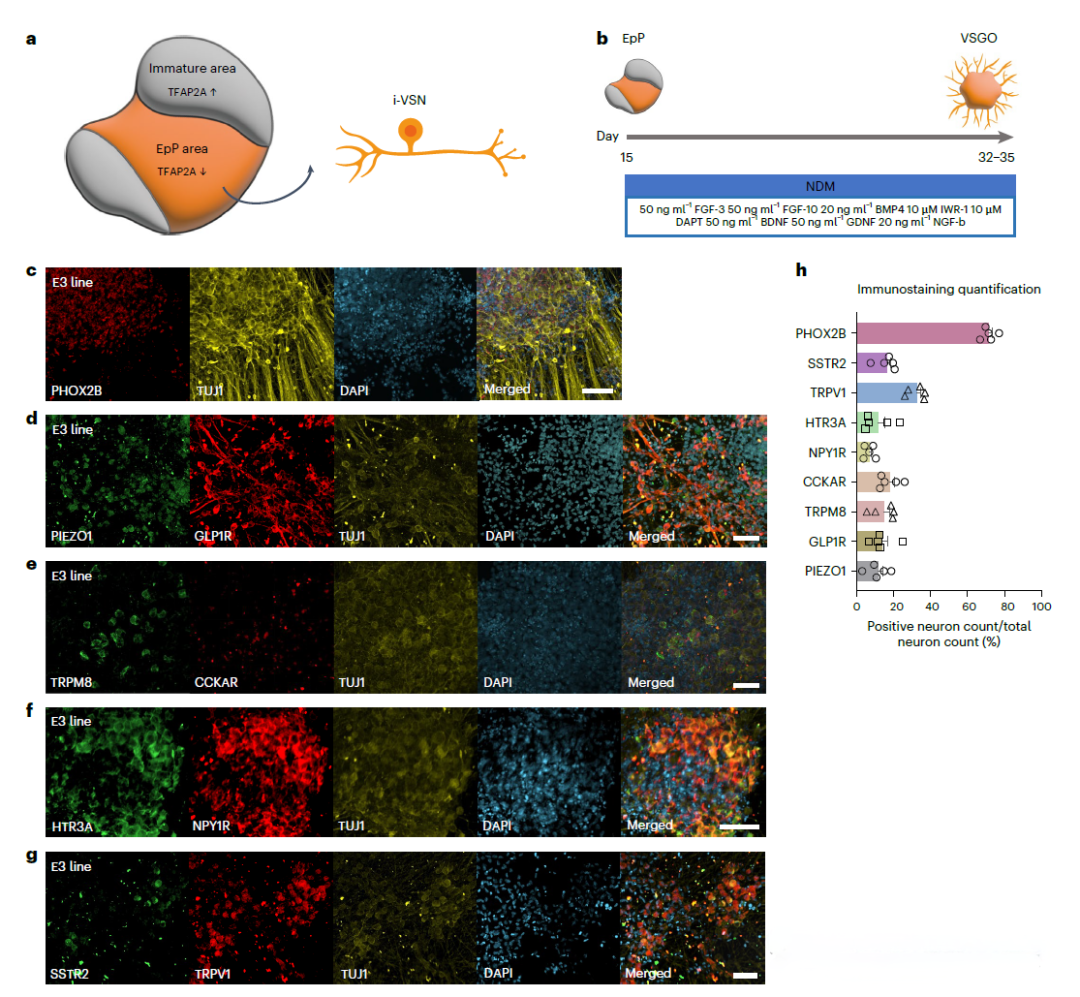
Using this model, the researchers observed that VSGOs can express a variety of VSNs-specific molecular markers, and single-cell RNA sequencing analysis revealed heterogeneous molecular characteristics and development trajectories similar to native VSNs. In further experiments, by adding fluorescently labeled Aβ and tau proteins to HCOs and observing the spread of these proteins in VSGOs, it was found that these pathological proteins could spread through VSNs, and this process was affected by the APOE genotype. In particular, the presence of the APOE4 allele significantly enhances the uptake of Aβ and tau proteins by VSNs. In addition, by conducting experiments using iPSCs from patients with Alzheimer's disease, the researchers found that the transmission of Aβ and tau in these VSGOs was significantly correlated with patient clinical data, which further confirmed the reliability of VSGOs in simulating disease pathology.
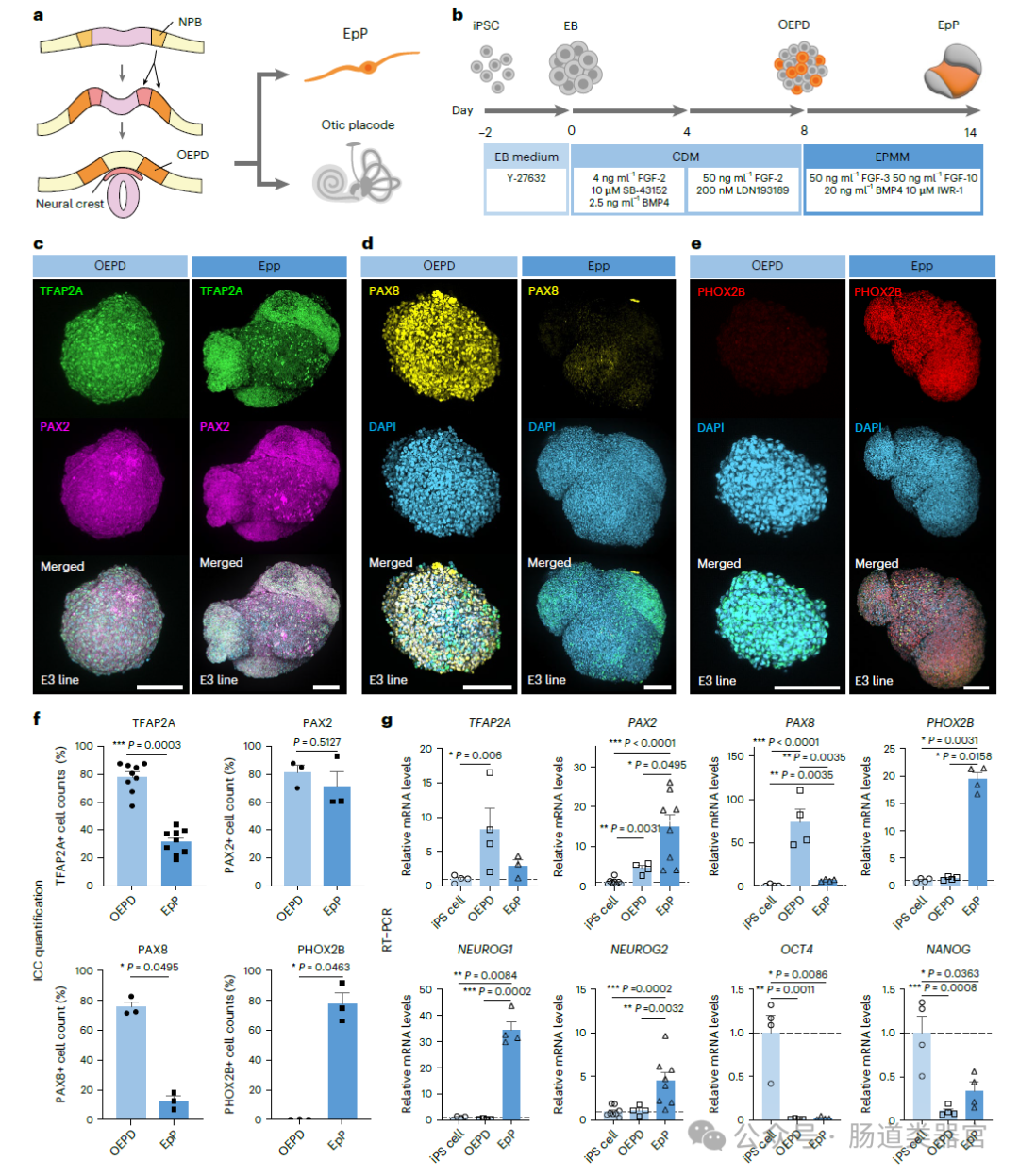
Expression of typical VSN markers in VSSYS differentiated from EpP
In this study, the organoid culture steps were carefully designed to ensure that induced pluripotent stem cells (iPSCs) could efficiently differentiate into visceral sensory ganglionic organoids (VSGOs) and colon organoids (HCOs).
The steps for cultivating VSGOs are as follows:
·Induction of epigill plate (EpP) formation: First, iPSCs are induced to form the auric-epigill precursor domain (OEPD) through specific media. This step involves the use of specific growth factors and inhibitors, such as FGF and BMP, to promote the formation of OEPD.
·Differentiation into VSGOs: Subsequently, OEPD is further induced to differentiate into EpP, which in turn differentiates into VSGOs. In this process, the production of VSN is promoted by adjusting FGF, Wnt and BMP signaling pathways, as well as adding neurotrophic factors such as β-NGF, BDNF and GDNF.
·Maturation of VSGOs: VSGOs are matured in a specially designed medium that supports neuron survival and functional expression until the required level of maturity for the experiment is reached.
The steps for cultivating HCOs are as follows:
·Differentiation of iPSCs: iPSCs are differentiated into intestinal epithelial cells that mimic intestinal development through the regulation of a series of growth factors and signaling pathways.
·Formation of HCOs: differentiated intestinal epithelial cells form HCOs through self-organization. These organoids can simulate the function and structure of the intestine in vitro.
The purpose of cultivating these organoids is to establish an in vitro model to study the role of the gut-nerve-brain axis in neurodegenerative diseases.
By integrating VSGOs and HCOs on a microfluidic chip, the researchers built a three-dimensional "axis-chip" system to simulate signal transmission from the gut to the brain. This system can not only simulate the interactions of VSNs with the gut, but also study how these interactions affect the brain, especially in disease states. For example, by adding labeled Aβ and tau proteins to HCOs, researchers were able to observe how these pathological proteins spread to the brain through VSNs and explore their role in diseases such as Alzheimer's disease (AD). In addition, the model allows researchers to assess the impact of different genetic backgrounds (such as APOE genotypes) on the spread of pathology and explore potential treatment strategies. In this way, research provides a powerful platform to deeply understand and simulate complex biological processes and open up new paths for future drug development and disease research.
Use a microfluidic chip to create a 3D on-chip shaft connecting VSGO and HCO
This study creatively applied induced pluripotent stem cell (iPSCs) differentiation technology to build visceral sensory ganglion organoids (VSGOs), and combined these organoids with colon organoids (HCOs) on a microfluidic chip, successfully simulating key links of the gut-nerve-brain axis. This in vitro model provides a powerful tool for exploring the interaction between the gut and the brain and its impact in neurodegenerative diseases.
This study not only demonstrates the potential function of VSGOs in delivering gut-derived pathological proteins to the brain, but also emphasizes the role of APOE genotypes in pathological transmission, providing a new perspective on the pathogenesis of diseases such as Alzheimer's disease (AD). In addition, this research provides an important experimental platform for future drug development and exploration of treatment strategies. Despite the limitations of research, its results have undoubtedly deepened our understanding of complex biological processes and opened up new avenues for the study of related diseases.