
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic joint inflammation and progressive bone destruction.
Although existing drugs (such as methotrexate, biologics) can relieve symptoms, some patients still face insufficient efficacy or side effects.
In recent years, mesenchymal stromal cells (MSCs) have become a hot topic in RA treatment research due to their unique immunoregulation and tissue repair capabilities. This article will focus on analyzing the clinical mechanism and research progress of MSCs.
MSCs regulate key cells in RA pathology by directly contacting or secreting active substances such as exosomes and cytokines:
1. Inhibits the invasiveness of fibroblast-like synovial cells (RA-FLS)
RA-FLS is one of the "culprits" that cause joint destruction. Its abnormal proliferation and migration can aggravate pannus formation and cartilage degradation. Studies have found that umbilical cord derived MSCs (UC-MSCs) can deliver miRNAs (such as miR-140- 3p, miR-451a) through exosomes, inhibit the expression of pro-inflammatory factors (IL-1β, TNF-α) and matrix metalloproteinases (MMP) in RA-FLS, thereby reducing tissue damage. In addition, MSCs can also down-regulate cadherin 11 (CDH11) levels and block the pro-inflammatory signaling between RA-FLS and immune cells.
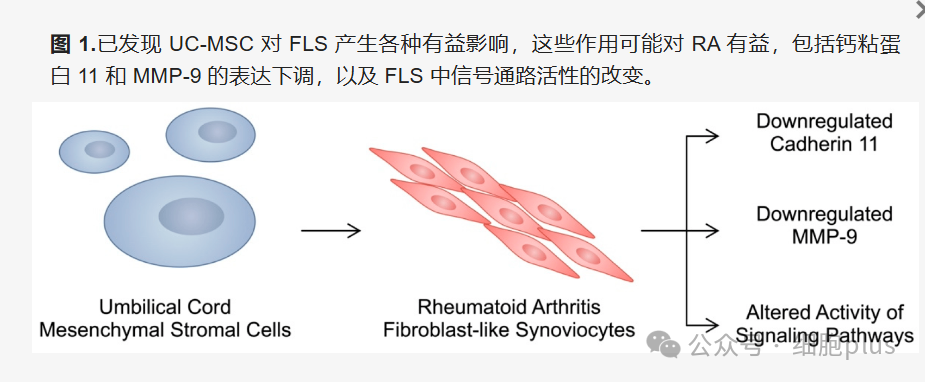
2. Regulate macrophage polarization
M1-type macrophages in the synovial membrane secrete large amounts of pro-inflammatory factors such as TNF-α and IL-6, accelerating the inflammatory cascade. By releasing IL-6 and TGF-β1, MSCs promote the transformation of M1-type macrophages into anti-inflammatory M2-type macrophages, reduce osteoclast formation, and delay bone erosion. Animal experiments have shown that MSC treatment can significantly reduce local TNF-α levels in joints and improve pathological scores.
3. Balancing T cell subsets
Th17/Treg cell imbalance is the core pathological mechanism of RA. Preclinical studies have shown that MSCs inhibit Th17 differentiation and promote Treg proliferation by secreting prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO), thereby reducing pro-inflammatory factors such as IL-17 and IFN-γ, while increasing anti-inflammatory mediators such as IL-10 and TGF-β. For example, in clinical trials, UC-MSCs can increase the proportion of Treg in patients 'peripheral blood and significantly decrease Th17-related indicators.
Multiple animal and human trials have verified the safety and potential efficacy of MSCs:
· Animal models have clear effects
In collagen-induced arthritis (CIA) mice, intravenous injection of UC-MSCs significantly reduced joint swelling, reduced IL-6 and TNF-α levels, and the efficacy was comparable to methotrexate. Histological analysis showed that the degree of joint destruction in the treatment group was similar to that in the healthy control group.
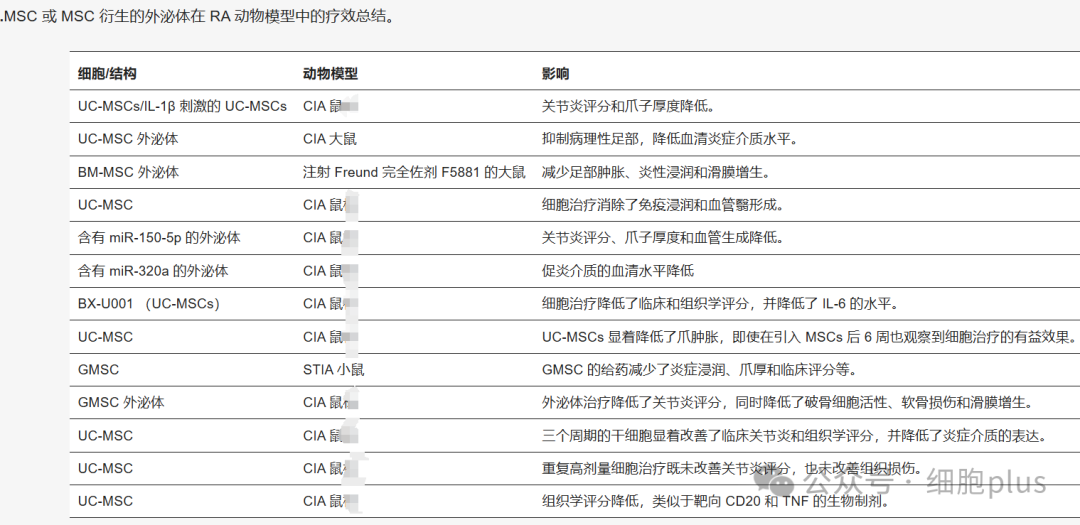
·Clinical trials are beginning to show potential
A clinical study in patients with RA showed that after 3 months of combined treatment with UC-MSCs and conventional antirheumatic drugs (DMARDs), the patient's DAS28 score, C-reactive protein (CRP) and rheumatoid factor (RF) levels were significantly reduced, and there were no serious adverse reactions. Long-term follow-up (3-year) data showed that joint function and quality of life continued to improve.


·Exploration of new drug delivery strategies
Research tried to load anti-inflammatory ingredients such as curcumin into MSC exosomes and found that its inhibitory effect on synovial cell activity is better than a single drug, providing a new idea for targeted therapy.
In summary, MSCs regulate the inflammatory network of RA through multiple targets, providing a new option for refractory patients. With the development of technologies such as gene editing and exosome engineering, more precise and lasting therapeutic effects are expected to be achieved in the future.