
Parkinson's disease, as the second most common neurodegenerative disorder worldwide, is characterized by the progressive degeneration and death of dopaminergic neurons in the substantia nigra of the midbrain as its core pathological mechanism. This pathological process directly leads to a significant decrease in the level of dopamine neurotransmitters in the striata, which in turn causes typical motor symptoms in patients, including resting tremor (such as "pill-rolling" hand tremors), bradykinosis (such as difficulty in initiating movements and reduced stride), muscle rigidity (such as increased resistance during passive limb movement), etc., seriously affecting the quality of life of patients.
With the vigorous development of the field of regenerative medicine, the rise of stem cell therapy has brought brand-new ideas for the treatment of neurodegenerative diseases. This type of therapy, relying on the self-renewal ability and multi-directional differentiation potential of stem cells, is expected to achieve the replacement and functional reconstruction of damaged nerve cells through directional induction and differentiation into dopaminergic neurons. Meanwhile, the neurotrophic factors secreted by stem cells (such as brain-derived neurotrophic factors, glial cell-derived neurotrophic factors, etc.) can also provide a protective microenvironment for the remaining neurons and delay the progression of the disease. This treatment strategy that approaches from the perspective of neural repair provides an important possibility for breaking through the limitation of traditional drugs that can only alleviate symptoms and achieving intervention at the pathological level of Parkinson's disease.

In April 2025, the top academic journal Nature published two independent clinical studies simultaneously. These two studies focused on the application of stem cell therapy in the treatment of Parkinson's disease, conducting in-depth explorations on human induced pluripotent stem cells (IPscs) and human embryonic stem cells (HESCs) respectively, and evaluating their safety in the treatment of Parkinson's disease.
The research results show that the transplantation of dopaminergic neuron precursor cells derived from iPS cells and hES cells is expected to become a safe and promising treatment method for Parkinson's disease.


Phase I/II trials of dopaminergic precursor cells derived from IPscs
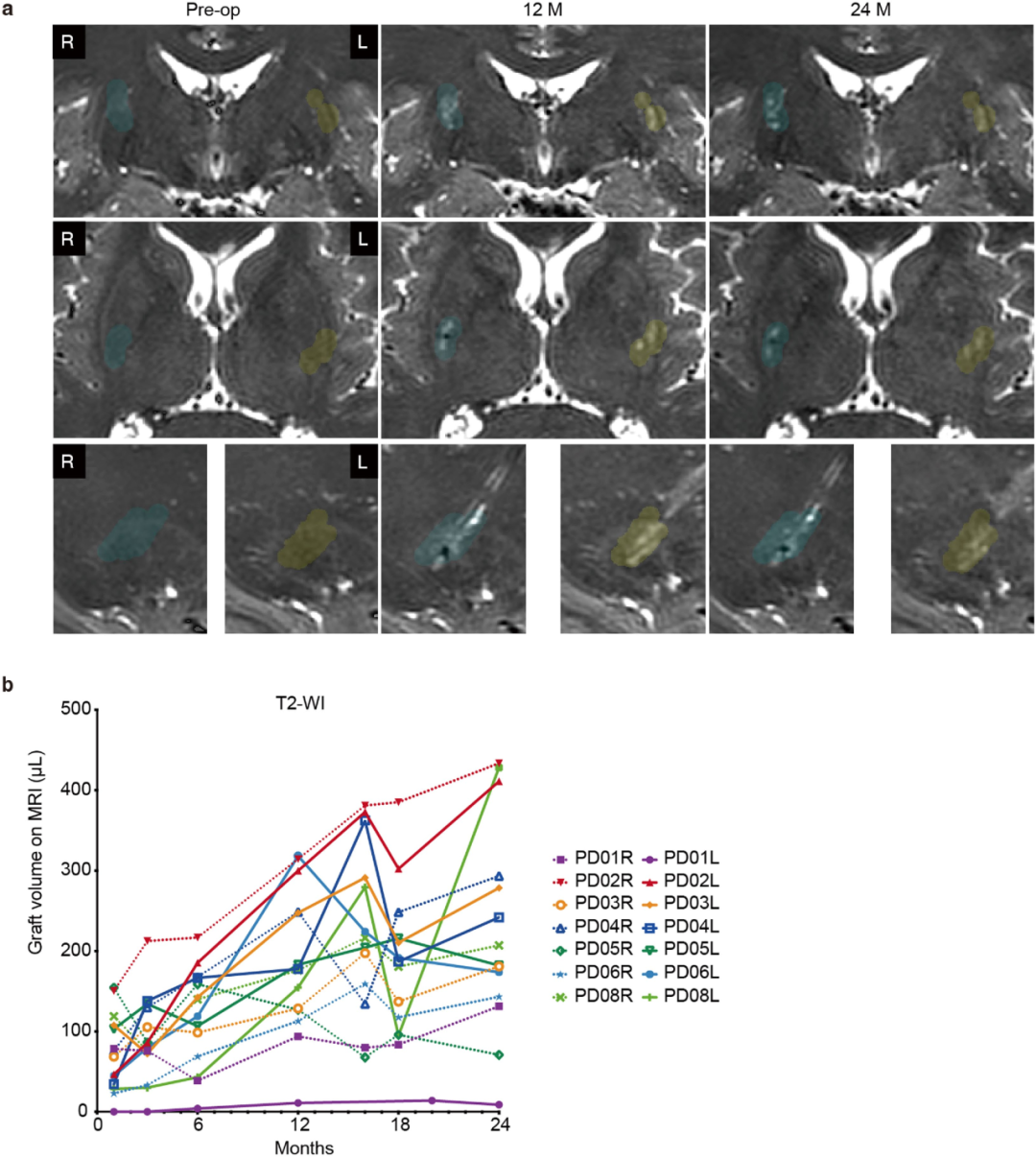
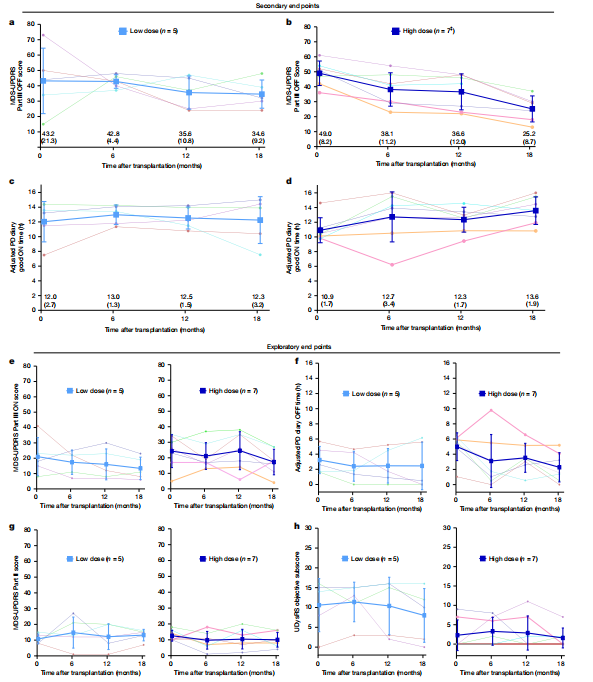
A single-center open-label Phase I/II clinical trial conducted by a research team from Kyoto University used HLA-matched human induced pluripotent stem cell (hiPSC) -derived CORIN+ dopaminergic precursor cells to perform bilateral putamen transplantation on 7 Parkinson's disease patients aged 50-69 years (low dose: 2.1-2.6 ×10⁶ cells/side;) High dose: 5.3-5.5×10⁶ cells/side, and a 24-month follow-up was conducted.
The trial results showed that the treatment safety and tolerability were good, and no serious adverse events or tumor formation occurred. Although some patients experienced mild aggravation of motor disorders due to drug adjustment, no rejection reaction was observed even after discontinuation of the immunosuppressive drug (tacrolimus) for 15 months. In terms of therapeutic effect evaluation, among the 6 patients, the motor symptoms (MDS-UPDRS score) of 4 patients improved significantly. Imaging showed that the intake of ¹⁸F-DOPA in the putamen area increased by 44.7%, and the increase in dopamine synthesis in the high-dose group was more obvious.
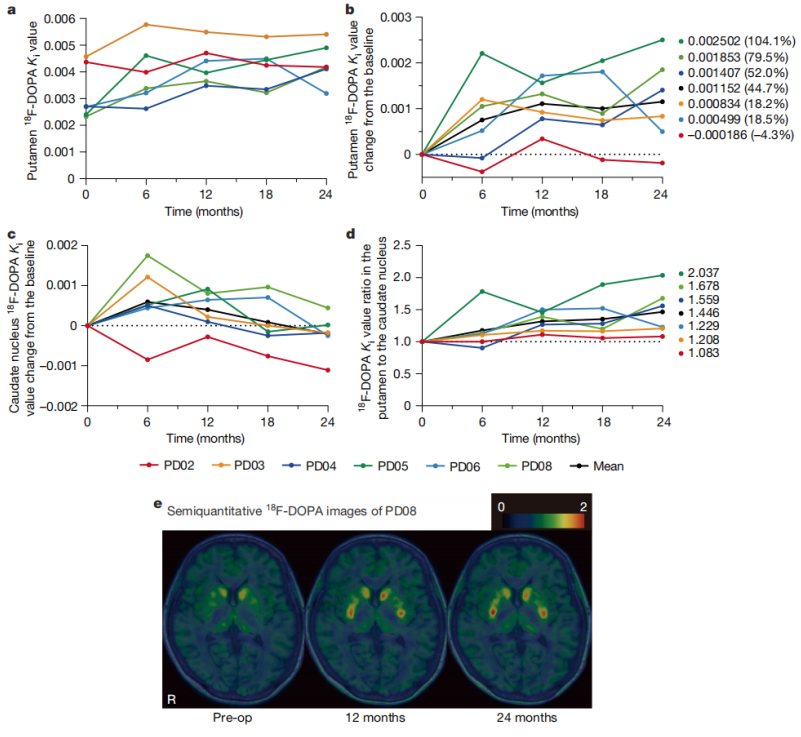
Detection of DA synthesis by 18F-DOPA PET
The research team stated that they will subsequently expand the sample size to conduct Phase III clinical trials and explore the optimization of immunosuppressive regimens, with the aim of promoting the early clinical transformation of this technology.
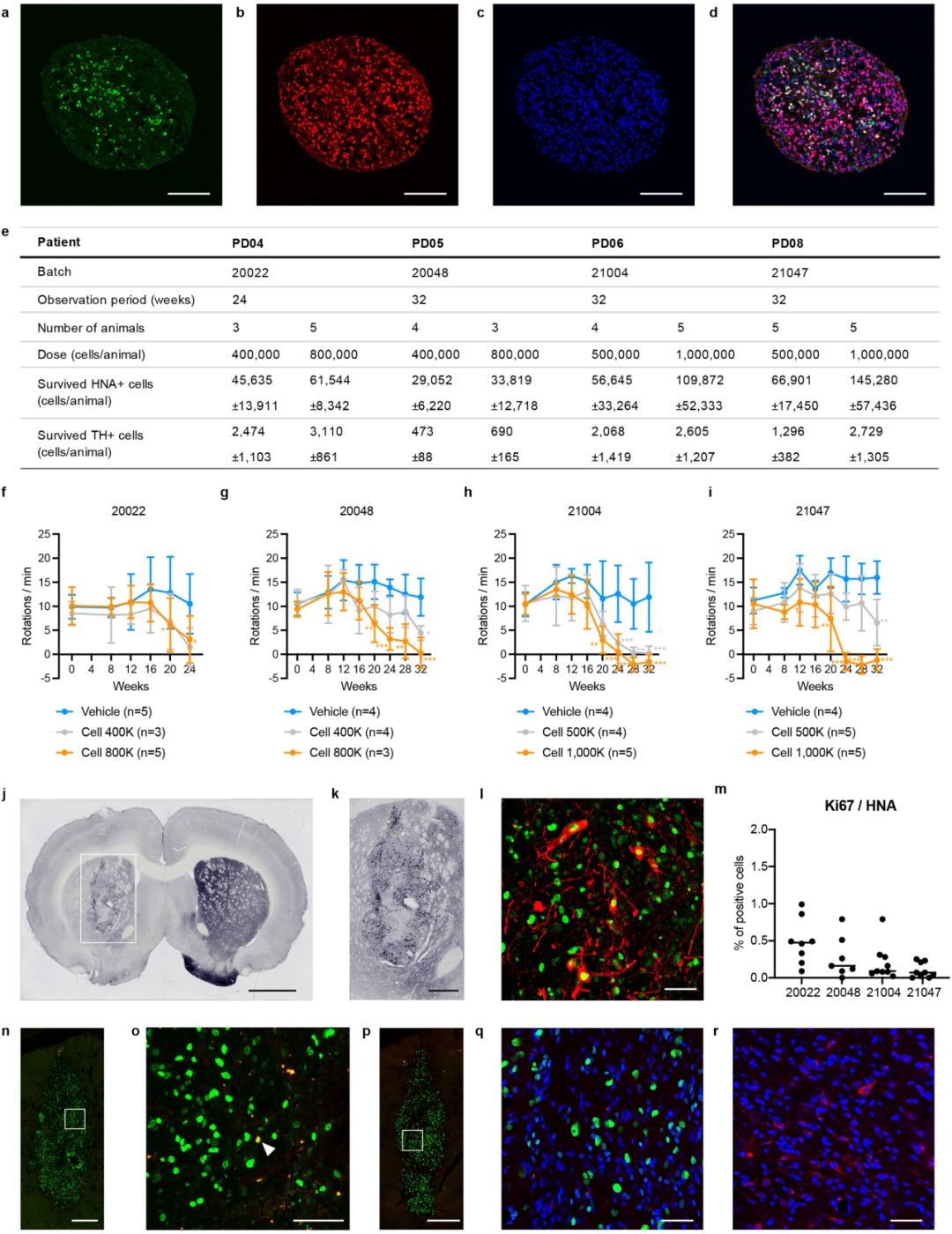
The same donor cells used by the patients were transplanted into the rat PD model
Cell survival of IPSC-derived DA progenitor cells
Phase I trial of dopaminergic neurons derived from hESC
In the open-label Phase I clinical trial jointly conducted by Memorial Sloan Ketterine Cancer Center and BlueRock Therapeutics (a wholly-owned subsidiary of Bayer), dopaminergic precursor cells derived from human embryonic stem cells (hESC) were used to transplant bilateral putamen regions in 12 patients with Parkinson's disease. Among them, 0.9×10⁶ cells were implanted on each side in the low-dose group, and 2.7×10⁶ cells were implanted on each side in the high-dose group.
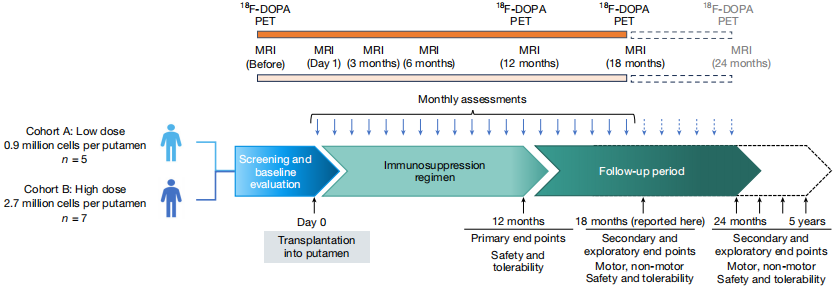
Summary of Research Design
The primary endpoint of the trial showed that no serious adverse events related to grafts or immunosuppression occurred within 12 months, and no tumor or graft-induced dyskinesia (GID) was found during the 18-month follow-up. Imaging examinations revealed an increase in the uptake of ¹⁸F-DOPA in the putamen area through PET, which was more significant in the high-dose group. In terms of clinical scores, the MDS-UPDRS III OFF score in the high-dose group improved by an average of 23 points compared with the baseline (decreased by 35%). The patient received immunosuppressive therapy with tacrolimus combined with glucocorticoids one year after the operation, but did not maintain it for a long time.
The research team believes that this cell therapy has good safety, providing strong support for the subsequent deterministic clinical trials.
Clinical results 18 months after transplantation
Both of these clinical trials have confirmed the safety of allogeneic (non-autologous) transplantation of stem cell-derived cell products for the treatment of Parkinson's disease and have shown preliminary efficacy. In the future, larger-scale, double-blind controlled trials are still needed to verify the long-term efficacy and optimize the cell dose, transplantation strategy and patient selection.
Regenerative medicine enterprises have also made certain progress in the field of Parkinson's disease
In November 2024, Huixin Medical Valley conducted a 12-month postoperative efficacy evaluation on the first Parkinson's disease patient who received iNSC-DAP (induced neural stem cell-derived dopaminergic neural precursor cells) treatment. This patient had suffered from Parkinson's disease for 8 years before the operation, had severe motor complications and was unable to take care of themselves in daily life. After the treatment, the patient was able to stand and walk independently one year after the operation. The posture stability was significantly improved and the quality of life was significantly enhanced. Imaging analysis showed that the transplanted dopaminergic nerve cells successfully survived in the patient's brain and released neurotransmitters, repairing the damaged neural function. Furthermore, no serious adverse reactions occurred in the patient after the operation, further confirming the safety and significant efficacy of this treatment.
On December 20, 2024, the CDE approved the clinical trial of UX-DA001 injection (human midbrain dopaminergic neural precursor cell injection), a cell candidate drug derived from induced pluripotent stem cells (IPscs) by Yuesai Biotech for the treatment of primary Parkinson's disease. This injection is based on Yuesai Biotech's innovative pluripotent stem cell technology platform, which includes a high-throughput stem cell differentiation lineage tracing platform, an efficient neural differentiation technology platform, and an iPSCs reprogramming technology platform. It can differentiate human midbrain dopaminergic neural precursor cells with high purity, high stability, and high efficiency.
On January 14, 2025, Bayer and its clinical-stage cell therapy subsidiary BlueRock Therapeutics announced that they would initiate a Phase III clinical trial of bemdaneprocel, an investigational cell therapy for Parkinson's disease. The registration clinical trial named ExpITE-2 is expected to start in the first half of 2025 and will be the first phase III trial for evaluating allogeneic pluripotent stem cell-derived therapies for Parkinson's disease. bemdaneprocel is an investigational allogeneic pluripotent stem cell-derived therapy composed of dopaminergic neurons generated by pluripotent stem cells, which is surgically implanted into the brains of Parkinson's disease patients. After transplantation, these cells may reshape the neural networks damaged by Parkinson's disease, thereby restoring the patient's motor and non-motor functions.
In April 2025, the first-class new drug, the universal IPSC-derived dopaminergic neural precursor cell injection (XS411 cell Injection), submitted by Shize Biotech, was approved for clinical trials in China for the treatment of primary Parkinson's disease. Previously, in January 2025, the US FDA officially approved the drug to conduct a registered Phase I clinical trial for the treatment of Parkinson's disease in the United States. The FDA also granted Shize Biotech a special Exemption for the new Parkinson's disease drug.
On May 7, 2025, Aspen Neuroscience, an American biotech company, announced the 6-month follow-up data of the first three patients in the ASPIRO Phase I/IIa clinical trial of its autologous cell therapy drug ANPD001 for Parkinson's disease. The results were encouraging. This therapy performs excellently in terms of safety, tolerability and early efficacy.
The functional cure of Parkinson's disease has always been a difficult problem in the field of neural regeneration, while stem cell transplantation is gradually becoming a breakthrough method for the treatment of Parkinson's disease. Stem cells can differentiate into dopaminergic nerve cells and be transplanted into specific areas of the patient's brain to replace dead neurons and rebuild the damaged neural network. This "cell replacement" therapy is expected to achieve functional cure and significantly improve the motor function and quality of life of patients. Meanwhile, the development of AI technology has also provided new impetus for the treatment of Parkinson's disease. In the future, with the optimization of stem cell transplantation technology and the further development of AI technology, the treatment of Parkinson's disease will become more efficient and personalized, bringing new hope to patients.