
Although the central nervous system (CNS) is an immune-exempt organ, clinical experience in fetal substantia nigra transplantation for Parkinson's disease (PD) shows that immunomodulatory therapy is needed in nerve transplantation. Fetal substantively nigra transplantation derived from aborted fetuses was first applied in clinical practice in the 1980s as an open trial and in two double-blind placebo-controlled trials in the United States. In some early open-access trials, drugs such as cyclosporine, steroids and azathioprine were used in combination to suppress immune rejection, referring to organ and bone marrow transplantation protocols. In the two subsequent double-blind trials conducted in the United States, immunosuppressive therapy was significantly reduced. One trial did not use immunosuppressive therapy at all, while the other only used cyclosporine for six months. However, the results of these double-blind studies did not meet their endpoint indicators. Based on these clinical experiences and the results of animal studies, it is suggested that cell therapy for the central nervous system requires a certain degree of immunosuppression. Meanwhile, some long-term research reports show that even if immunosuppression is discontinued several years ago, the graft can still survive and function for more than 10 years. However, fetal donor tissues are limited, and stem cell-based cell therapy has broad potential. Compared with fetal tissues, stem cell donors have higher purity, better homology, are immature and have lower immunogenicity.
At present, the immunomodulatory protocol for cell therapy using pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), in the central nervous system has not yet been established. Despite this, clinical treatment of Parkinson's disease based on pluripotent stem cells has been initiated. Cell therapy based on stem cells can be divided into autologous transplantation and allogeneic transplantation. In the brain, autologous nerve grafts can survive without immunosuppression, and there is a case of cell therapy for Parkinson's disease based on autologous IPscs. On the other hand, allogeneic transplantation usually has a mismatch of human leukocyte antigen (HLA), which increases the risk of graft rejection. Clinically HLA-homozygated induced pluripotent stem cell (iPSC) libraries can theoretically achieve HLA-matched transplantation, thus reducing the possibility of an expected immune response. Previous studies have shown that in the transplantation of MHC-mismatched IPSC-derived dopaminergic neural precursor cells (iPSC-DANs) in non-human primates, the use of tacrolimus alone can control the immune response. Then, whether this immunomodulatory protocol is applicable in clinical practice requires further exploration.
Recently The team of Asuka Morizane from Kyoto University in Japan published an article titled "Control of immune response in iPSC-based allogeneic cell therapy clinical" in the journal CellStemCell trialfor Parkinson's disease reported the first clinical trial (jRCT2090220384) that used allogeneic IPSC-differentiated dopaminergic neural progenitor cells (iPSC-DANs) transplantation to treat patients with Parkinson's disease, and only tacrolimus was used for immunotherapy. The results showed that no clinically significant immune response was observed regardless of whether the HLA was matched or not. However, the highly sensitive mixed lymphocyte response using dendritic cells derived from IPscs as stimulants revealed that lymphocytes in HLA-mismatched transplant recipients were activated. This discovery indicates that the low expression of HLA in iPSC-DANs contributes to its successful implantation in the immune-privileged central nervous system. These results suggest that stem cell transplantation targeting the central nervous system may only require moderate immunosuppressive therapy.

To be used in clinical practice, researchers established an iPSC library by screening healthy volunteer donors with homozygally HLA-A, HLA-B and HLA-DR. In this clinical trial, the cell line with the most common HLA haplotype in the Japanese population was used. This cell line is homozygous at the six loci of HLA-A, B, C, DR, DQ and DP, and is estimated to match approximately 16.9% of the Japanese population. To detect immunogenicity, researchers analyzed the expression of HLA class I and Class II in the QHJI01s04 cell line (HLA homozygous iPSCs). Compared with the control PBMCs and IPSC-derived DCS, the basal expression level of HLAI classes in iPSC nerve cells was lower, and exposure to the pro-inflammatory cytokine IFN-γ would up-regulate the expression of HLAI classes. The expression of HLAII classes was almost undetectable, and exposure to IFN-γ also showed almost no upregulation. After cell reinfusion therapy for 7 patients, oral tacrolimus was administered for immune tolerance. For PD01, immunosuppression persisted for up to 12 months after the second surgery. The dose is halved and then discontinued 12 to 15 months after the operation (after the second surgery in PD01 cases). The trough value of tacrolimus and the time course of estimated glomerular filtration rate (eGFR) indicated that the administration of tacrolimus was well controlled in all subjects, and no serious adverse reactions of renal insufficiency occurred. No other adverse events caused by immunosuppression occurred, including opportunistic infections. It confirmed the feasibility and tolerability of tacrolimus in terms of immune control after treatment.
To monitor potential graft-induced immune responses, positron emission tomography (PET) was performed on activated microglia using the transport protein (TSPO) ligand [¹⁸F] GE180 during immunosuppression at 3, 6, and 12 months after transplantation. No uptake of [¹⁸F] GE180 was shown in any region, indicating that there was no significant inflammation during immunosuppression. No changes occurred even after the discontinuation of tacrolimus. Magnetic resonance imaging (MRI) also did not detect any significant signs of inflammation caused by the graft throughout the trial period. The detection of inflammatory cytokines and donor-specific antibodies in the serum showed no clinically significant immune response. Using iPSC-DC as the stimulant, the mixed lymphocyte response (MLR) of transplant patients was detected. It was found that the MLR of HLA-mismatched patients was higher than that of matched patients. It indicates that the low expression of HLA in iPSC-DANs contributes to its successful implantation in the immune-privileged central nervous system.
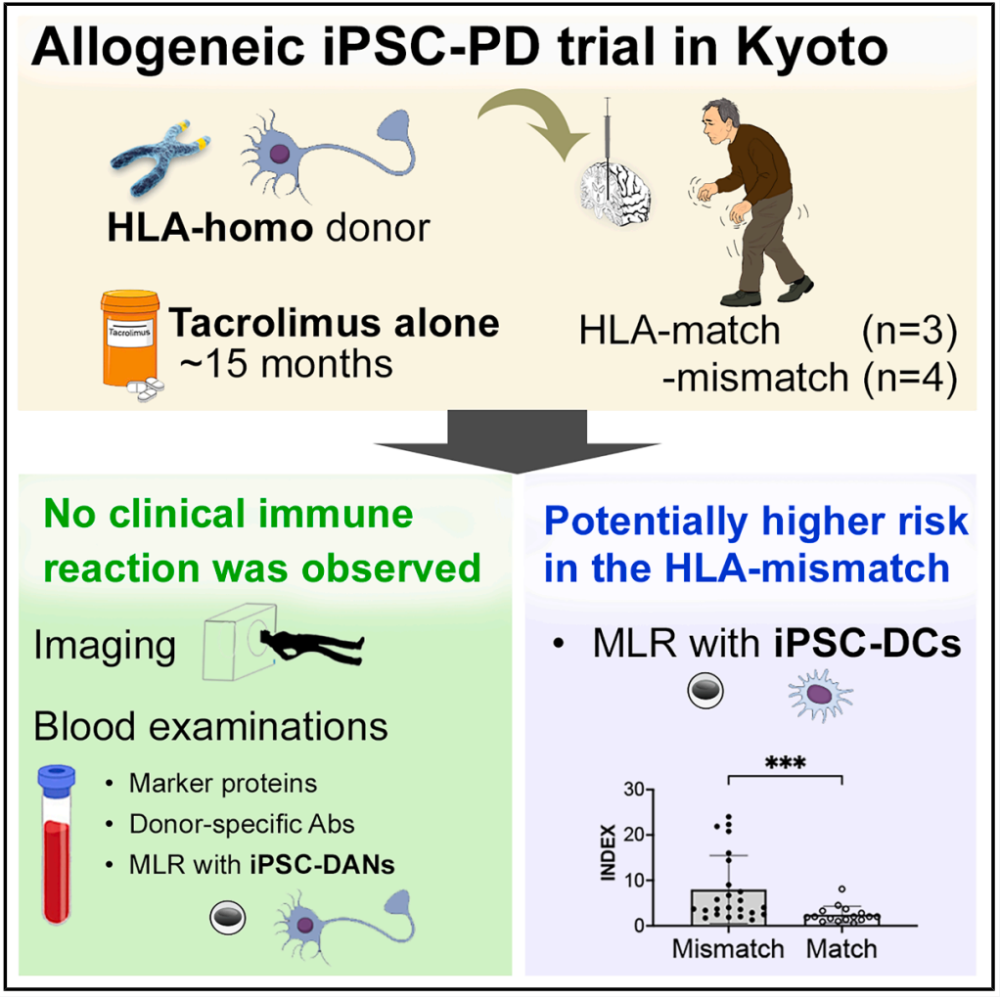 In conclusion, IPSC-derived dopaminergic neural precursor cells were used for cell transplantation treatment in patients with Parkinson's disease, and tacrolimus was used for immunosuppressive therapy. The results showed that the donor cells survived in vivo regardless of whether the HLA was matched or not, and no clinically significant immune response occurred.
In conclusion, IPSC-derived dopaminergic neural precursor cells were used for cell transplantation treatment in patients with Parkinson's disease, and tacrolimus was used for immunosuppressive therapy. The results showed that the donor cells survived in vivo regardless of whether the HLA was matched or not, and no clinically significant immune response occurred.
Original link:https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(25)00269-3