
Source: Cell World
Liver transplantation is the only effective treatment for acute liver failure, end-stage liver disease, primary liver cancer and some hereditary liver diseases, but there is a large gap in the supply of livers relative to the actual demand. Therefore, many patients and their families have to wait for the liver source, which is very painful.
As an alternative to organ transplantation, hepatocyte transplantation has attracted much attention. Hepatocyte transplantation has achieved some remarkable results, but on the other hand, the number of primary human hepatocytes (PHHs) commonly used in hepatocyte transplantation is small and difficult to expand in vitro. So the researchers turned to human pluripotent stem cells (hPSCs), which can expand indefinitely and differentiate into any type of cell, including liver cells.
Next, we will look at the clinical application prospects of human pluripotent stem cells to hepatocyte differentiation.
01 Disease modeling
hiPSCs obtained from patient biopsies can differentiate into hepatocytes in vitro, which we call PSC-derived hepatocyte-like cells (PSC-HLCs), which can be used for in vitro modeling of some genetic diseases. For example, scientists have successfully modeled genetic diseases such as alpha-1 antitrypsin deficiency and cystic fibrosis by using hiPSC-HLCs to build organoids [1]. In addition to single-gene mutation diseases, the combined treatment of iPSC-HLCs organoids with free fatty acids can also simulate the process of lipid accumulation and fibrosis in non-alcoholic fatty liver disease.
02 Toxicological study
HLCs can retain some characteristic phenotypes of donor cells, and can model rare phenotypes repeatably. It also has the metabolic capacity of PHH and the proliferation and repeatability of cancer cell lines. Therefore, it has a good application prospect to use HLCs as a liver model for toxicological studies in vitro. It is important to note that the toxic reactions observed in vivo are mediated by complex interactions between different cell types, the use of single hepatocyte cultures to simulate drug-induced hepatotoxicity is limited, and co-culture HLCs with supporting cells can extend the maintenance of their liver function to some extent.
03 Drug screening
Drug screening, that is, for the development of drugs that can selectively interact with genes and gene products, or drugs that can interfere with specific molecular mechanisms so that these drugs can be used in human clinical trials. The application of knowledge of pharmacokinetics and pharmacodynamics is crucial in drug screening. In the process of screening using 2D models, phenomena such as plastic adsorption may affect the relationship between dose and drug effect. In contrast, three-dimensional spheres, organoids and organ chips are more suitable for studying the pharmacokinetic curves of drugs.
04 Bioartificial liver
The principle of bioartificial liver is to exchange the patient's plasma with human liver cells in the bioreactor through cardiopulmonary bypass, replace the liver for a short time, and promote the regeneration and repair of the damaged liver, thus helping some patients with liver failure to restore liver function. Bioartificial liver requires a large number of liver cells to maintain hemodialysis and liver function in patients, and PSC-HLCs can be used as a biological component in it, but their low long-term viability and high production cost hinder their rapid application.
05 Tissue engineering
The use of hiPSC-HLCs compatible with genes or human leukocyte antigens can overcome the limitations of regenerative medicine due to the limited number of healthy donor tissues and the risk of tissue rejection. One approach is to inoculate hiPSCs on acellular human liver extracellular matrix scaffolds for differentiation. Compared with standard differentiation conditions, the use of liver acellular scaffolds leads to upregulation of liver function markers, and the expression levels of some transcription factors and nuclear factors are comparable to those of adult PHH [2]. Another approach is to use PSC-derived organoids [3]. However, for organoid transplantation, how to prepare scalable, reproducible and stable organoid models after cryopreservation is the final key challenge. A recent study reported that HESC-derived organoids can be expanded for 20 generations and can stably retain the phenotypic characteristics of bipotent precursor cells [4]. After transplantation in mice, they can differentiate into functional hepatocytes or bile duct epithelial cells and show significant rebreeding capacity.
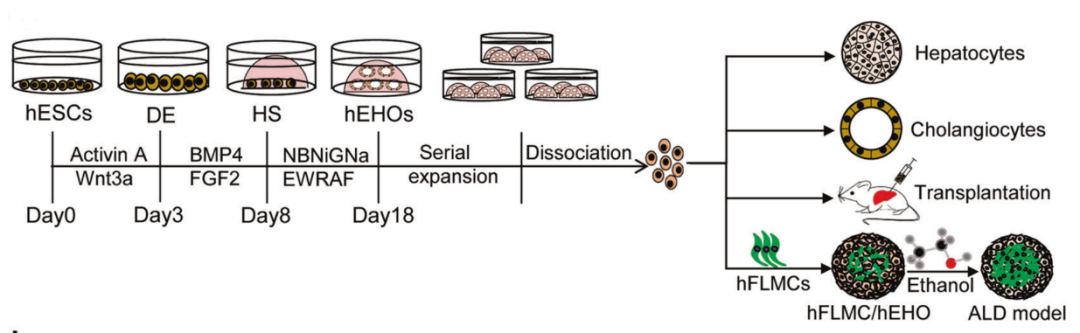
FIG. 1 Induced hESCs to gradually differentiate into hepatocytes by simulating embryonic liver development
In addition, researchers heterotopic transplanted liver buds composed of HipSCs-derived cells into immunodeficient mice, and found that the liver buds quickly connected to the blood vessels of mice and began to function normally after 10 days, and the liver buds transplanted into mice were metabolically similar to adult livers. The researchers transplanted liver buds into ALF mouse models and found that ALF mice had improved survival compared to controls, suggesting that transplanted liver buds have the ability of liver self-repair and can serve as a bridge to therapy.
It can be expected that as the application research of human pluripotent stem cells to hepatocyte differentiation continues to go deeper, it will bring more breakthroughs and hopes to people.
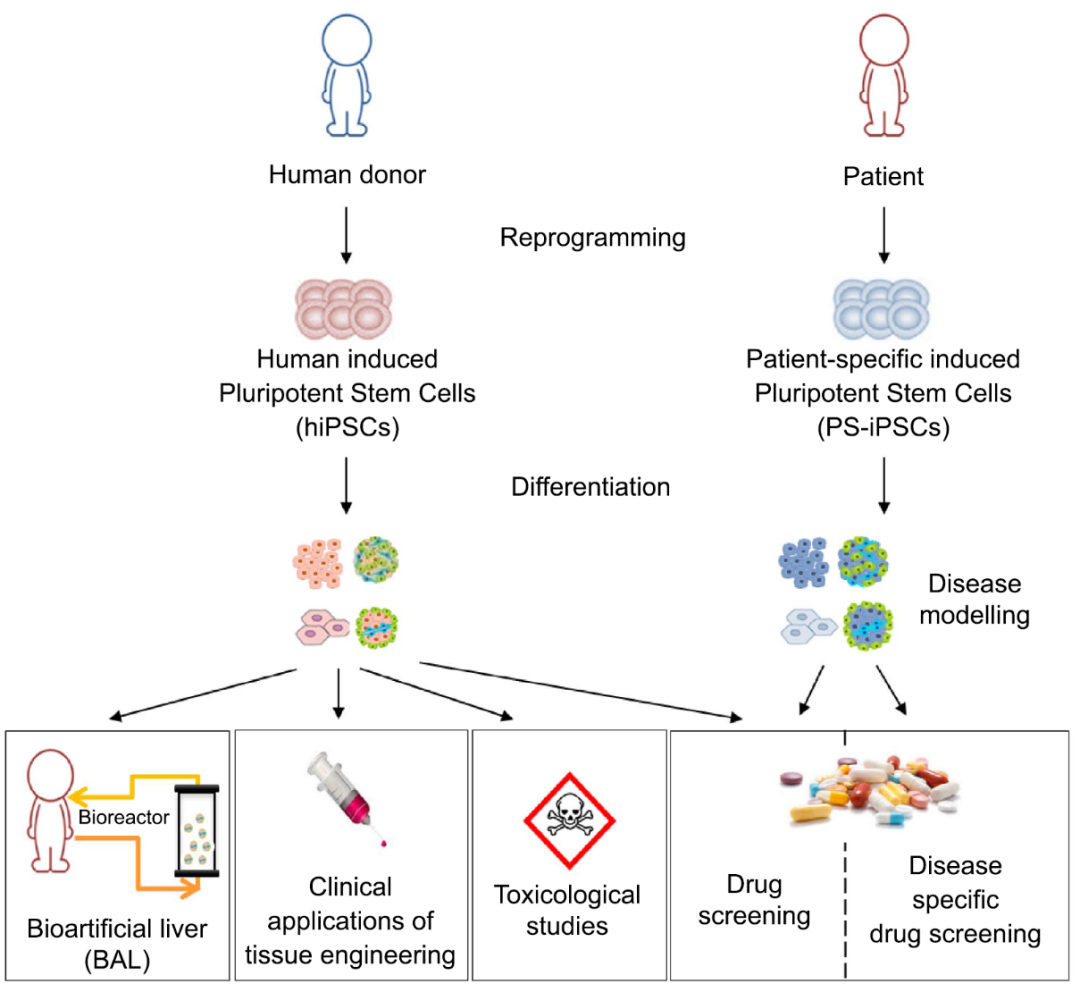
 FIG. 2 Application of hPSC differentiation into hepatocytes
FIG. 2 Application of hPSC differentiation into hepatocytes
References:
1. Sampaziotis, F., et al., Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol, 2015. 33(8): p. 845-852.
2. Jaramillo, M., et al., Decellularized human liver extracellular matrix (hDLM)-mediated hepatic differentiation of human induced pluripotent stem cells (hIPSCs). J Tissue Eng Regen Med, 2018. 12(4): p. e1962-e1973.
3. Takeishi, K., et al., Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells. Cell Rep, 2020. 31(9): p. 107711.
4. Wang, S., et al., Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res, 2019. 29(12): p. 1009-1026.
5. Takebe, T., et al., Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep, 2017. 21(10): p. 2661-2670.
6. Luce, E., et al., Advanced Techniques and Awaited Clinical Applications for Human Pluripotent Stem Cell Differentiation into Hepatocytes. Hepatology, 2021. 74(2): p. 1101-1116.