
Source:eMedClub
Compared with CAR-T cells, CAR-NK cells have attracted much attention due to their multiple killing mechanism, higher safety and wide range of sources. Current studies have proved the safety and feasibility of CAR-NK cell-mediated immunotherapy, which has become a convenient and effective cancer treatment.

Unlike the killing mechanism of T cells, NK cells can initiate the killing process simply by recognizing the target cells. The killing activity of NK cells is regulated by both inhibitory and activator receptors on the cell surface. When the balance between activation and inhibition is broken, NK cells will perform corresponding functions. When sufficient inhibitory ligands are expressed on the surface of target cells, the function of NK cells is inhibited. However, the killing function of NK cells can be activated when the expression level of active ligand on the surface of target cells is up-regulated or the expression level of inhibitory ligand on the surface of target cells is down-regulated.
NK cells kill target cells through three main mechanisms: 1. Directly kill target cells by releasing cytoplasmic particles containing perforin and granase; 2. 2. Release cytokines, such as IFN-γ, TNF-α, etc. to induce apoptosis of tumor cells through interaction with corresponding receptors on the surface of tumor cells; 3. The Fc receptor CD16 binds to the Fc segment of the antibody and can trigger mediated antibody-dependent cytotoxicity (ADCC) to kill cells.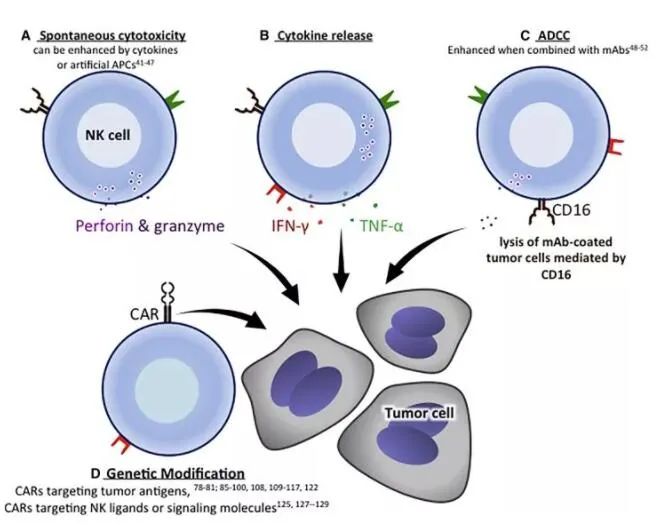
▲ Mechanisms of tumor cytotoxicity mediated by NK cells
Induced pluripotent stem cells (IPscs) were formed by the introduction of transcription factors into cells by Shinya Yamanaka in 2006. After combining this technology with NK cells, the derived iPSC-NK has a more mature phenotype and effective cytolytic activity, and IPscs provide a homogeneous CAR-NK cell population that can be expanded to clinical scale. As a result, CAR-iNK cells have become the signature "existing" product used in cancer immunotherapy.
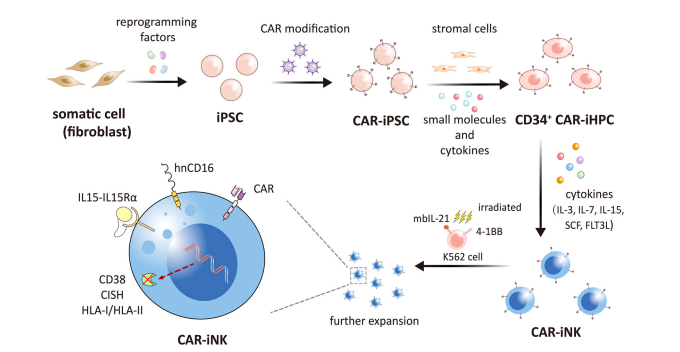
▲ iPSC衍生的CAR-NK细胞的产生
Strategies for enhancing iPSC-NK cell function are as follows:
Converged builds enhance security
IL-15 is necessary for NK cell survival, but during NK cell culture can cause peripheral NK cell expansion and induce IFN-γ expression, resulting in systemic toxicity. Therefore, the researchers took advantage of the easy genetic modification of IPscs and introduced specific fusion constructors (IL-15RF) into IPscs to further enhance the function of NK cells by modifying IPscs, thus enhancing the toxicity and persistence of iPSC-NK cells.
Introduction of CD16a improves survival
NK cell-mediated ADCC is regulated by the receptor CD16a, and the high affinity, non-lysable receptor CD16a is introduced into iPSC, which improves the resistance of IPSC-NK cells to activation induced CD16a lysate and ADCC effect, and significantly improves the survival rate of malignant hematological diseases and solid tumor xenotransplantation models.
Gene editing tools
CD38, as a bifolateral extracellular enzyme, has the activity of cyclase and hydrolase at the same time, and widely acts on the surface of a variety of immune cells. Through specific gene editing, NK cells have strong innate immune function, ADCC, persistence, and metabolic and transcriptional characteristics related to adaptive NK cells, showing significant anti-tumor activity. All kinds of evidence support the further development of iPSC-NK cells for tumor immunotherapy in medical institutions at home and abroad.
How can CAR engineering be used to further improve tumor targeting and cytotoxicity of iPSC-NK cells
CAR-iNK cell therapy
Chimeric antigen receptors can recognize specific antigens on tumor cells and activate downstream signaling, which has been developed to further improve cytotoxic function and anti-tumor effect of NK cells, while avoiding risks, CAR iNK cell therapy also retains the anti-tumor activity of CAR T cell therapy. In the case of xenotransplantation, less cytokine is released and overall anti-tumor activity is higher in vivo and in vitro. The clinical efficacy of CAR-iNK cells has greatly encouraged researchers at home and abroad, and clinical trials of CAR-iNK cells from various sources have been launched, expecting CAR-iNK cell therapy to bring immune cell therapy into a new era.
iPSC cells were constructed with CAR for immunotherapy
iPSCs can be genetically engineered together with CAR (CAR-ipscs). At present, most CAR structures used in NK cells are designed to enhance the activation and proliferation of T cells. In order to optimize the function of such NK cells, a foreign research institution designed and screened an NK-cell-specific CAR structure (NK-CAR). Compared with CAR T cells, NK-CAR-modified iPSC-NK (NK-CAR-iNK) cells can effectively inhibit growth and prolong survival in ovarian cancer xenotransplantation models, avoiding weight loss, organ damage, or cytokine release syndrome in patients. These results suggest that NK-CAR-iNK cell therapy is safer and more effective than conventional CAR-T therapy. Therefore, suitable CAR constructs are indispensable for improving the anti-tumor ability of CAR-INK cells.
CAR-iNK cells in a multifunctional device
In addition to using CAR to design IPscs, many research institutions are also trying other directions of gene modification to further improve the function of CAR-INK cells, and a foreign institution has created a triple gene modified CAR-INK (iDuo-NK) cells to show a durable response in lymphoma and leukemia. To further explore the use of CAR-iNK cells in multiple myeloma, the agency also designed a four-fold gene-edited CAR-iNK cell, called an iDuo-MM-CAR-NK cell. iDuo-MM-CAR-NK cells showed sustained and long-lasting tumor inhibition both in vivo and in vitro, and its tumor inhibition effect was comparable to that of BCMA-targeted CAR T cells, but there were no complications such as GVHD. More importantly, when combined with Isatuximab (anti-CD38 monoclonal antibody), iDuo-MM-CAR-NK cells maximized ADCC activity and had significant anti-tumor effects on MM.
Recently, Century Therapeutics developed a CAR-iNK cell product with six genetic modifications designed to improve durability, functionality, and safety. Phase I clinical trials using this CAR-iNK cell therapy for the treatment of relapsed or refractory differentiated CD19-positive B-cell malignancies are being recruited to evaluate its safety, pharmacokinetics, and initial efficacy.
The challenges of CAR-iNK cell therapy in clinical application
The production of IPscs under conditions of cGMP compliance is necessary to meet clinical norms. Somatic cells used to derive IPscs are first obtained and subjected to associated infectious disease pathogens or disease tests (RCDAs), followed by CAR-iNK cells that must be generated through a robust, repeatable, and CGMP-compliant manufacturing process.
Strategies to improve the antitumor activity of CAR-iNK cells in solid tumors
In recent years, many preclinical and clinical studies have demonstrated the feasibility and effectiveness of CAR-iNK cells for hematological malignancies. However, the efficacy of CAR-modified immune cells in solid tumors is hampered by tumor antigen heterogeneity, tumor invasion limitation, and immunosuppressive tumor microenvironment (TME). Over the past few years, several approaches have been developed to improve the expansion, persistence, resistance to TME, and metabolic adaptation of CAR-T/NK cells, which can also be used for CAR-iNK cell therapy.
Improve the expansion and persistence of CAR-iNT cells
As an important cytokine for NK cell proliferation and maintenance, IL-15 has been widely used to improve the persistence and cytotoxicity of CAR-iNK cells. In addition to IL-15, memory-like [ML] NK cells induced by IL-12 and IL-18 cytokines also exhibit enhanced antitumor activity and ameliorative effects in vivo. In addition, IL-21 also contributes to the proliferation and maturation of NK cells. The researchers also found that knocking out HLA-I and II in IPscs evades the recognition of allogeneic T and NK cells, thus promoting the sustainability of IPSC-based regenerative medicine applications.
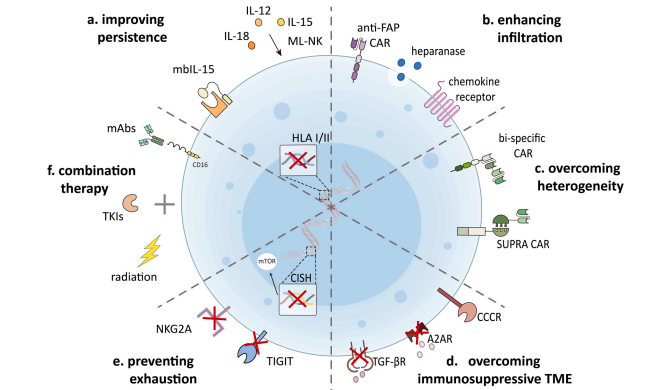
▲ 增强CAR-iNK细胞活性的策略
To enhance the infiltration of CAR-iNK cells in solid tumors
Insufficient infiltration of CAR-iNK cells in solid tumors is an important factor limiting the therapeutic effect. The researchers refer to the strategy that CAR-T cells secrete stroma-degrading enzymes such as heparinase to promote tumor invasion and anti-tumor activity of CAR-T cells for CAR-iNK cell therapy to enhance their delivery to different solid tumors.
Increase tumor targets of CAR-iNK cells to overcome tumor heterogeneity
Antigen loss and tumor heterogeneity are important factors leading to cancer recurrence, and targeting multiple antigens to cancer cells is one of the ways to prevent antigen loss after cell therapy with CAR. In addition to combination therapy with CAR T cells that target different antigens, many institutions have developed bisspecific cars. For example, the multi-antigen initiation and killing recognition pathway mediated by synthetic Notch receptors developed abroad achieves complete and controllable tumor cell killing by targeting antigens that are homogeneous but not absolutely tumor-specific. These techniques may provide a similar protocol for CAR-iNK cells to successfully target heterogeneous tumors while minimizing toxicity both inside and outside the tumor.
Genetically engineered CAR-iNK cells overcome immunosuppressive Tumor microenvironment (TME)
CAR-iNK cells must face adverse metabolic conditions including hypoxia, acidic pH, and low blood sugar levels, as well as immunosuppressive cells that can impair NK cell function. One of the most important immunosuppressive cytokines, transforming growth-β (TGF-β), is hypoxic and often impacts NK cell function in a variety of ways. CAR-iNK cells with TGF-β antibodies or inhibitors are expected to improve NK cell resistance to immunosuppressive TME. In addition to TGF-β, adenosine is another key immunosuppressive metabolite that limits the activation of cytotoxic lymphocytes while impairs the anti-tumor immune response. Ablating adenosine signaling or administering an A2A adenosine receptor (A2AR) antagonist can enhance NK cell-mediated anti-tumor immunity. Thus, blocking or switching these immunosuppressive signaling pathways in CAR-iNK cells could help overcome immunosuppressive TME.
Regulates metabolic fitness of CAR-iNK cells and prevents their depletion
Cell metabolism plays a key role in regulating NK cell function. The researchers improved metabolic fitness by activating the mTOR signaling pathway, thereby enhancing the expansion and anti-tumor function of iPSC-NK cells. In addition, inhibitory receptor upregulation also contributes to the immune tolerance of NK cells, but these strategies to improve CAR-iNK cell function need to be further validated in clinical trials.
Application of CAR-iNK cell therapy in combination therapy
CAR-iNK cells can be used in combination with TKIs, radiotherapy, and monogram-lowering antibodies to further enhance their anti-tumor effects.
Sum up
The success of CAR-mediated immunotherapy in hematologic malignancies and some solid tumors has driven the development of CAR-iNK cells for cancer immunotherapy. The advantages of IPscs provide many opportunities to enhance the function of CAR-modified IPSC-derived NK cells, making them a sharp edge against hematological malignancies and solid tumors. In addition, IPscs come from a wide range of sources and have considerable proliferation potential in vitro reprogramming, which significantly reduces production costs and makes CAR-iNK cells more beneficial to clinical applications.
Reference materials:https://www.biospace.com